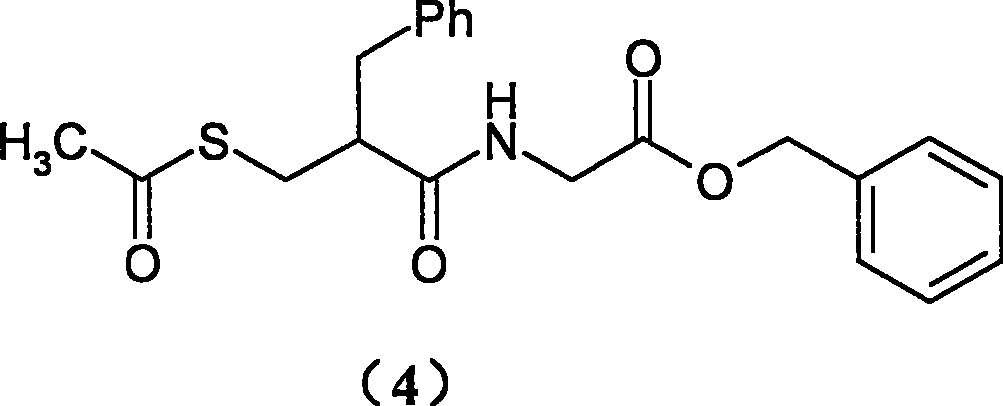
Preparation process of cadotril
A technology of thiomethyl and oxo substitution, applied in organic chemistry and other fields, can solve the problems of difficult removal of DCU and impractical industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
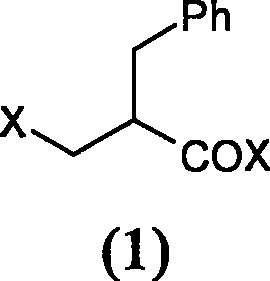
[0035] Preparation of 2-chloromethylphenylpropionyl chloride
[0036] I is halogenated with sulfoxide halide
[0037] Add 1.4ml (19.2mmol) thionyl chloride and 3 drops of DMF to a 25ml three-necked reaction flask, stir for 5 minutes, cool in an ice bath, add 1.0g (5.6mmol) 2-benzyl-3-hydroxypropionic acid, and keep The temperature was reacted for 30 minutes, and the temperature was raised to 60° C. for 1 hour. Thin layer chromatography judged that the reaction was complete, and the solution was evaporated to dryness under reduced pressure to obtain 1.0 g of a colorless oil.
[0038] II is halogenated with phosphorus halide
[0039] Add 2.0g (11.2mmol) of 2-benzyl-3-hydroxypropionic acid into a 50ml three-necked reaction flask equipped with a drying tube, then add 20ml of dichloroethane and stir to dissolve, cool to -10°C in an ice bath, and then add 3.5 gPCl 5 , maintained the temperature for 30 minutes, then raised the temperature to 60°C for 20 minutes, and the reaction w...
Embodiment 2
[0043] Preparation of 2-bromomethylphenylpropionyl bromide
[0044] Add 2.0g (11.2mmol) 2-benzyl-3-hydroxypropionic acid in 125ml three-necked reaction flask, add 10ml dichloromethane and 1% pyridine, stir to dissolve. Cool in an ice bath to -5°C, add 5.84 g (28 mmol) of thionyl bromide dropwise, maintain the temperature for 50 minutes, and evaporate the solution to dryness under reduced pressure to obtain 1.8 g of a colorless oil. MS(EI): 306(M + )
[0045] II Add 2.0g (4.5mmol) of 2-benzyl-3-hydroxypropionic acid into a 25ml three-necked reaction flask, add 10ml of dichloroethane, and stir to dissolve. After cooling in an ice bath to -10°C, add 4.5 g of boron tribromide, maintain the temperature for 30 minutes, and raise the temperature to 60°C for 20 minutes. Thin layer chromatography judged that the reaction was complete, and the solution was evaporated to dryness under reduced pressure to obtain a colorless oil 2.2g.
[0046] III Add 2.0g (4.5mmol) 2-benzyl-3-hydroxyp...
Embodiment 3
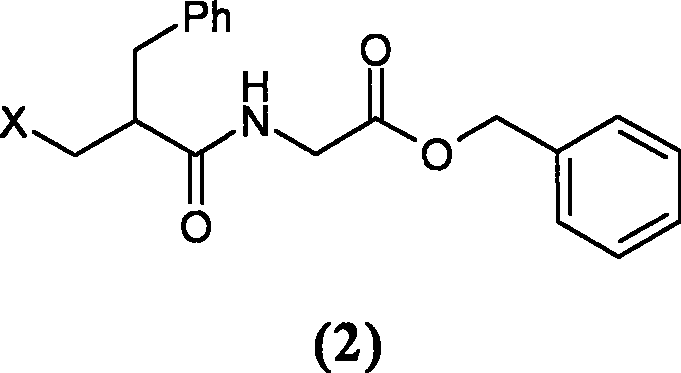
[0048] Preparation of N-(2-chloromethyl-1-oxo-3-phenylpropyl)glycine benzyl ester
[0049] Add 33.6g (166.8mmol) glycine benzyl ester hydrochloride and 15ml dichloromethane in a 100ml three-necked flask, add 40.0ml pyridine after cooling to 0°C in an ice bath, stir for 10 minutes, add dropwise 10ml to dissolve 22.8g (105.6mmol ) 2-chloromethylphenylpropionyl chloride. After 2 hours of reaction, the reaction was complete, washed twice with water, dried over anhydrous sodium sulfate, filtered, concentrated to dryness, stirred and crystallized with 200ml of petroleum ether to obtain 33.2g of solid (92.3% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com