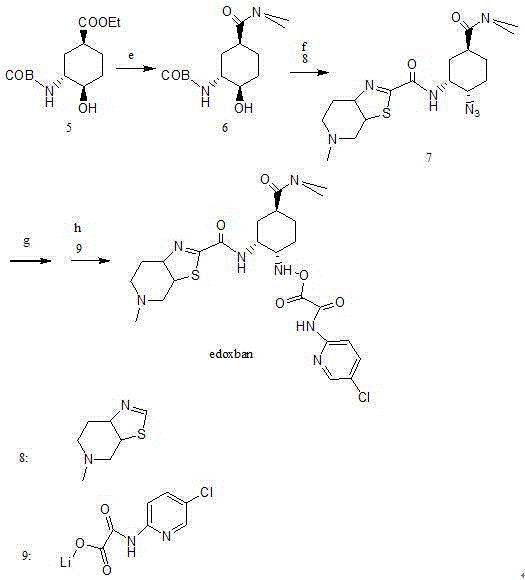
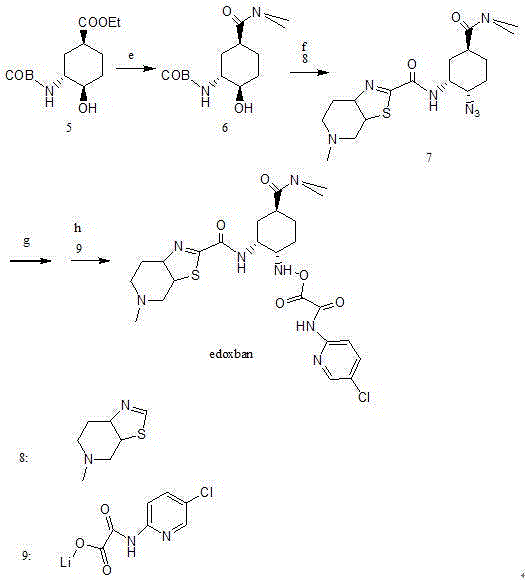
Edoxaban intermediate and preparation method thereof
A technology for edoxaban and intermediates, which is applied in the field of anticoagulant edoxaban intermediates and its preparation, can solve the problems of cumbersome post-processing, long steps for synthesizing products, and large solvent consumption, and achieve product quality Stable and reliable, mild reaction conditions, less waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0039] Example 1: the preparation of compound 2:
[0040] (1s)-3-cyclohexene-1-carboxylic acid (48g, 380mmol), dichloromethane (580ml), potassium iodide (82.1g, 494mmol), sodium bicarbonate (42.0g, 500mmol), water (530ml), cool down to 5°C, start stirring, slowly add I 2 (125.4g, 494mmol). After the addition, it was raised to 20°C and reacted for 3h. Add 800ml of 1N sodium thiosulfate solution to the reaction system, extract with 500ml of dichloromethane, wash the organic phase with sodium thiosulfate (300ml), water (500ml), saturated sodium chloride (300ml), anhydrous sulfuric acid Magnesium was dried, filtered, and distilled under reduced pressure to obtain a colorless solid 2 .
example 2
[0041] Example 2: Compound 2 Preparation of:
[0042] (1s)-3-cyclohexene-1-carboxylic acid (48g, 380mmol), dichloromethane (580ml), potassium iodide (82.1g, 494mmol), sodium bicarbonate (42.0g, 200mmol), water (530ml), cool down to 8°C, start stirring, slowly add I 2(125.4g, 494mmol). After the addition, it was raised to 25°C and reacted for 3h. Add 800ml of 1N sodium thiosulfate solution to the reaction system, extract with 500ml of dichloromethane, wash the organic phase with sodium thiosulfate (300ml), water (500ml), saturated sodium chloride (300ml), anhydrous sulfuric acid Magnesium was dried, filtered, and distilled under reduced pressure to obtain a colorless solid 2 .
example 3
[0043] Example 3: Compound 3 Preparation of:
[0044] compound 2 Add (89.3g, 354mmol) into a 2L four-neck flask containing absolute ethanol (810ml) solution, start stirring, slowly add 2N sodium hydroxide solution (213ml, 425mmol), stir and react at 20°C for 3h, and keep the reaction solution at Rotary evaporation under reduced pressure at 40°C, then add 500ml of water, extract once each with 500ml and 300ml of dichloromethane, wash the organic phase with 300ml of water, dry over anhydrous magnesium sulfate, filter, rotary evaporation under reduced pressure, and column purification (petroleum ether : ethyl acetate = 85: 15), to obtain a gray oil 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com