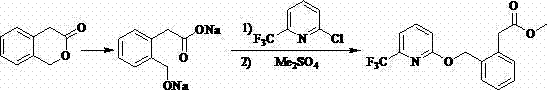A synthetic process of a green efficient agricultural fungicide
A technology of agricultural fungicides and synthetic processes, applied in organic chemistry and other directions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
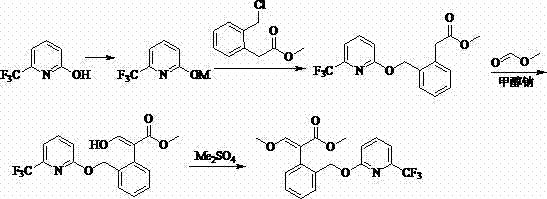
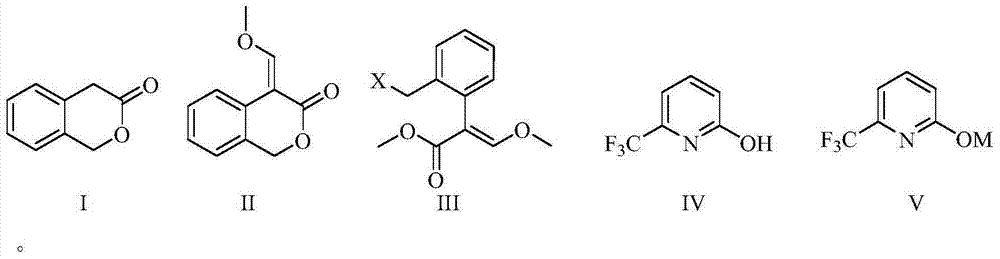
[0038] The synthesis process of a green and high-efficiency agricultural fungicide of this embodiment, as shown in Table 1, includes the following steps: 1) at 0°C, 30g (0.20 mol, 99%) of 3-isochroman Ketone (I) was added to a 1000 mL four-necked reaction flask, 200 mL of tetrahydrofuran was added thereto, 20.4 g (0.3 mol ) of sodium ethylate was added thereto under stirring, 17.8 g (0.24 mol ) of ethyl formate was added thereto, and the reaction 3 h, after the reaction was detected by the liquid phase, 150 mL of water was added thereto, adjusted to neutral with concentrated hydrochloric acid, extracted with 200 mL of chlorobenzene, separated from water, and 31.8 g of sodium carbonate (0.3 mol ), dimethyl sulfate 30.3g (0.24mol ), controlled temperature 0°C, reacted for 3 h, after the reaction was detected by liquid phase, 150 mL of water was added thereto, and the aqueous phase was extracted with chlorobenzene (3*30 mL) , combined the organic phases, and evaporated the solven...
Embodiment 2
[0042] The synthesis process of a green and high-efficiency agricultural fungicide in this embodiment, as shown in Table 1, includes the following steps: 1) at 40°C, 100 g (0.67 mol, 99%) of 3-isochroman Add ketone (I) to a 2000 mL four-necked reaction flask, add 400 mL of ethylene glycol dimethyl ether to it, add 82.5 g (0.74 mol) of potassium tert-butoxide to it under stirring, add phenyl formate to it 97.6 g (0.8 mol ), reacted for 4 hours, after the liquid phase detected the reaction was complete, added 150 mL of water to it, adjusted to neutral with concentrated hydrochloric acid, added 400 mL of methyl tert-butyl ether for extraction, separated water, and poured into the organic phase 117.3 g (0.85 mol ) of potassium carbonate and 93.0 g (0.74 mol ) of dimethyl sulfate were added to the solution, the temperature was controlled at 40°C, and the reaction was carried out for 4 h. Extracted with butyl ether (3*100 mL), combined the organic phases, and evaporated the solven...
Embodiment 3
[0046] The synthesis technique of a kind of green high-efficiency agricultural fungicide of the present embodiment, the step is roughly as embodiment 1, and its difference is: the mol ratio of alkyl formate and 3-isochromanone (I) is 1.5:1; the molar ratio of alkoxide anion source and 3-isochromanone (I) is 1.5:1; described step 1) in alkali and 3-isochromanone (I) ) in a mol ratio of 0.5:1; the mol ratio of a methylating reagent to 3-isochromanone (I) is 1.5:1; in the step 2), the halogenating reagent and 4-(methoxy The mol ratio of methenyl)-3-isobenzopyrone (II) is 1.5:1; the mol ratio of base and 2-hydroxyl-6-trifluoromethylpyridine (IV) in the step 3) is 1.5:1; other differences are shown in Table 1. Crude product recrystallization obtains 68.3 g picoxystrobin, mother liquor concentration continues crystallization and obtains 15.3 g picoxystrobin, totally 83.6 g, content 98.1% (external standard method detects), yield 82.7% (with starting material 3-isophenyl and dihydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com