Synthetic method of 16alpha-hydroxy prednisonlone
A technique for the synthesis of hydroxyprednisolone, which is applied in the fields of steroids and organic chemistry, and can solve the problems of long synthesis steps and complex post-processing of products, and achieve the effects of high efficiency, convenient post-processing, and high production capacity
Inactive Publication Date: 2010-11-03
无棣鑫岳化工集团有限公司
View PDF3 Cites 14 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In the existing synthetic route, the synthetic steps are long and the post-processing of the product is complicated
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
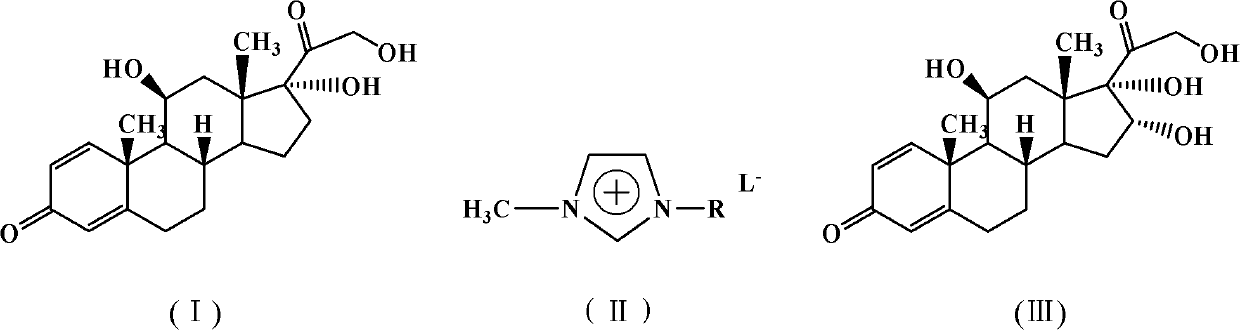
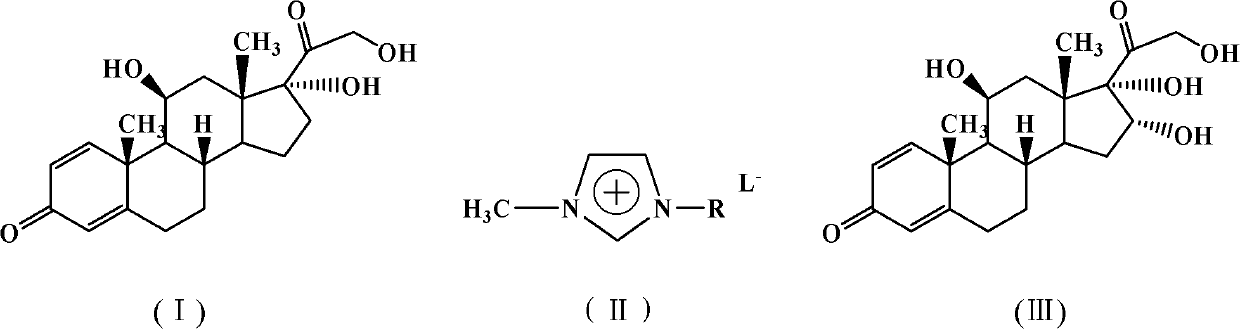
The invention discloses a synthetic method of 16alpha-hydroxy prednisonlone as shown in formula (III), comprising the following steps: taking the prednisonlone as shown in formula (I) as a raw material; carrying out dehydration reaction to generate a double bond in acidic ionic liquid as shown in formula (II); adding hydrogen peroxide aqueous solution to react; and adding water for further hydrolyzation after full reaction to finally obtain the 16alpha-hydroxy prednisonlone. The synthetic method of the invention adopts the property of the acidic ionic liquid as a reaction medium as well as a catalyst and changes technological conditions such as reaction time, reaction temperature and the like, thus achieving the purpose of reduced cost, energy conservation and emission reduction, and being applicable to industrial production.
Description
(1) Technical field The invention relates to a method for synthesizing 16α-hydroxyprednisolone. (2) Background technology 16α-Hydroxyprednisolone, chemical formula: C 21 h 28 o 6 , molecular weight: 376.44. It is an important intermediate of ciclesonide, a drug for treating asthma. Regarding the synthesis of 16α-hydroxyprednisolone, the methods reported at present all use prednisolone as a raw material, and form a ring with triethyl orthoacetate under the catalysis of pyridinium p-toluenesulfonate, and selectivity under weakly acidic conditions Ring-opening to obtain acetylated products, and then reacting with acetic anhydride under alkaline conditions to form diacetylated products, heating in the presence of potassium acetate to remove a molecule of acetic acid to obtain (11β)-hydroxy-21-acetoxy-1,4, 16-Pregnatriene-3,20-dione compound. The compound is selectively dihydroxylated under the action of potassium permanganate, and deacetylated in the presence of sodium hyd...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07J5/00
Inventor 裴文孙莉王海滨郑洁潘海燕胡香凝
Owner 无棣鑫岳化工集团有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com