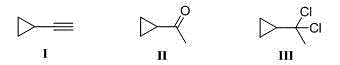
Method for efficiently preparing cylopropyl ethylnen
A technology of cyclopropylacetylene and cyclopropylethane, applied in the field of chemistry, can solve the problems of low condensation yield, low product purity, difficult separation, etc., and achieve the effects of mild reaction conditions, high atom utilization, and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1: Synthesis of intermediate 1,1-dichloro-1-cyclopropylethane (formula III)
[0049] Cyclopropylmethyl ketone (formula II): phosphorus pentachloride: catalyst is 1.0:1.0:1.0 according to the ratio of substances, cyclopropylmethyl ketone is 8.4g; phosphorus pentachloride is 20.8g; catalyst is methanol, and the mass of feed is 3.2g; the organic solvent is 8mL of dichloromethane, and its volume is 1 times the mass of cyclopropylmethyl ketone (mL / g).
[0050] In the reaction flask, add dichloromethane, turn on mechanical stirring, adjust the temperature to -10°C, and protect with nitrogen gas. Add phosphorus pentachloride and continue stirring. When the temperature of the feed liquid is stable, slowly add the mixture of cyclopropylmethyl ketone and methanol dropwise, and control the temperature of the feed liquid at -10 to 0°C. After the dropwise addition, the temperature was raised to 40°C, and samples were taken for gas phase monitoring every 1 h. After the rea...
Embodiment 2
[0053] Cyclopropylmethyl ketone: phosphorus pentachloride: catalyst is 1.0: 1.0: 0.4 feeding, cyclopropyl methyl ketone 8.4g; phosphorus pentachloride 20.8g; catalyst is isopropanol, feeding quality 2.4g ; The organic solvent is 34 mL of dichloromethane, and its volume is 4 times the mass of cyclopropylmethyl ketone (mL / g).
[0054] The reaction temperature was 0°C, and other operations were the same as in Example 1 to obtain 49.7 g of a dichloromethane solution of 1,1-dichloro-1-cyclopropylethane, of which 1,1-dichloro-1-cyclopropylethane The quality of alkane is 9.6g, and the yield is 69.1%.
Embodiment 3
[0056] Cyclopropylmethyl ketone: phosphorus pentachloride: catalyst is 1.0: 1.5: 1.2 feeding, cyclopropyl methyl ketone 8.4g; phosphorus pentachloride 31.2g; catalyst is isopropanol, feeding quality 7.2g ; The organic solvent is chloroform 42mL, and its volume consumption is 5 times of the mass of cyclopropylmethyl ketone (mL / g).
[0057] The reaction temperature was 25°C, and other operations were the same as in Example 1 to obtain 65.3 g of a chloroform solution of 1,1-dichloro-1-cyclopropylethane, in which 1,1-dichloro-1-cyclopropylethane The quality of alkane is 10.5g, and the yield is 75.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com