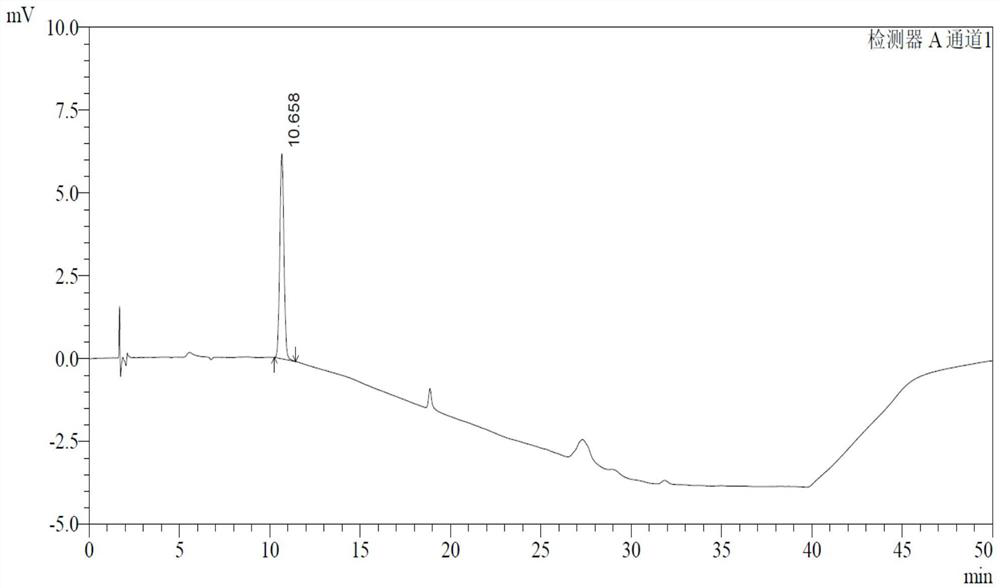
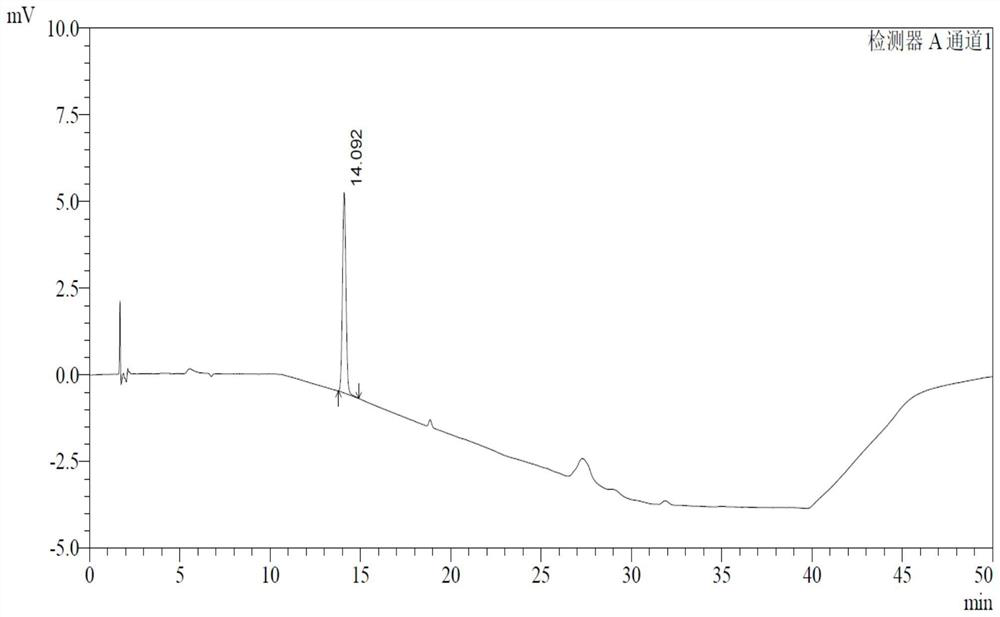
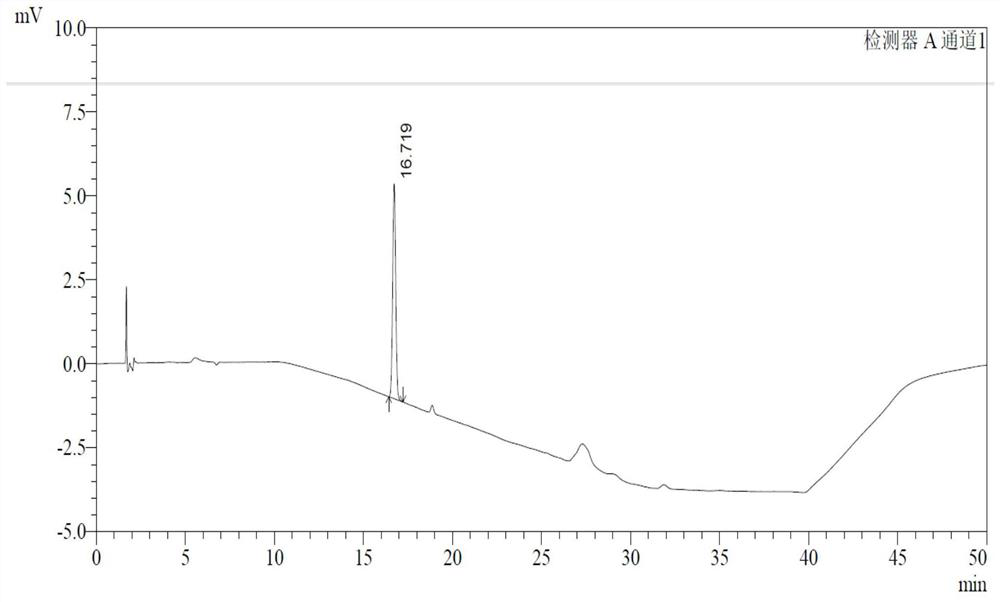
Determination method of p-toluenesulfonate esters in racecadotril
A technology of p-toluenesulfonate and racecadotril, which is applied in the field of medicine, can solve the problems of expensive GC-MS instruments, low penetration rate, complicated operation, etc., and achieve improved drug safety, good separation effect, exclusive strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1 Instruments and materials
[0044] 1.1 Instrument: Waters e2695 high performance liquid chromatography.
[0045] 1.2 Reagents: acetonitrile (chromatographic grade), potassium dihydrogen phosphate, phosphoric acid (reagent grade), and pure water.
[0046] 2 Methods and results
[0047] 2.1 Chromatographic conditions
[0048] Chromatographic column: Germany Merck LiChrospher RP 18 (end-capped, 250mm×4.0mm×5μm);
[0049] Mobile phase A: put 1.0g potassium dihydrogen phosphate in 1000ml water, adjust the pH to 2.5 with phosphoric acid;
[0050] Mobile phase B: acetonitrile;
[0051] Solvent: acetonitrile;
[0052] Detector: UV detector;
[0053] Detection wavelength: 225nm;
[0054] Flow rate: 1.0ml / min;
[0055] Column temperature: 40°C;
[0056] Injection volume: 10μl;
[0057] See Table 2 for gradient elution conditions.
[0058] Table 2 Gradient elution table
[0059] time (minutes) Mobile phase A(%) Mobile phase B(%) 0 60 40 5 60 ...
Embodiment 2
[0107] Embodiment 2: the detection of actual sample
[0108] 1) cancel the carcadotril bulk drug, dissolve with acetonitrile, be mixed with the solution that contains 20mg racecadotril in 1ml, as need testing solution;
[0109] 2) Dissolve the methyl p-toluenesulfonate reference substance, ethyl p-toluenesulfonate reference substance and isopropyl p-toluenesulfonate reference substance with acetonitrile, and prepare 1ml containing 0.1 μg methyl p-toluenesulfonate reference substance, A solution of 0.1 μg ethyl p-toluenesulfonate reference substance and 0.1 μg isopropyl p-toluenesulfonate reference substance;
[0110] 3) Get the need testing solution prepared in step 1) and the reference solution in step 2), adopt the same instruments and reagents as in Example 1, and detect according to the chromatographic conditions shown in step 1) 2.1.
[0111] 4) According to the external standard method, calculate the content of methyl p-toluenesulfonate, ethyl p-toluenesulfonate and iso...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com