Preparation method of racecadotril
A technology of racecadotril and acetyl mercapto, which is applied in the field of preparation of racecadotril, can solve the problems of low yield and low purity, achieve less reagents, simple process and high atom economy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
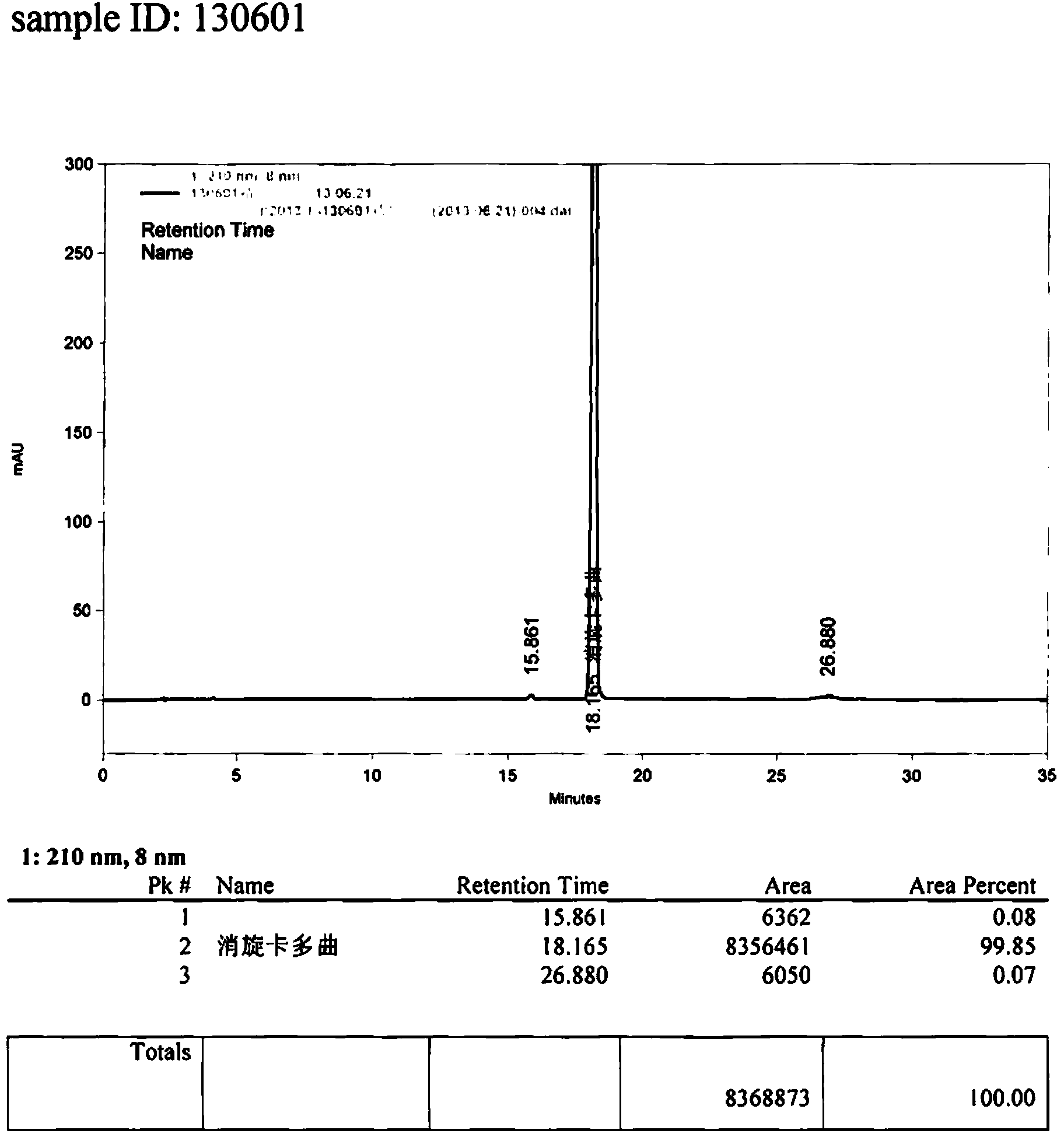
Image
Examples
Embodiment 1
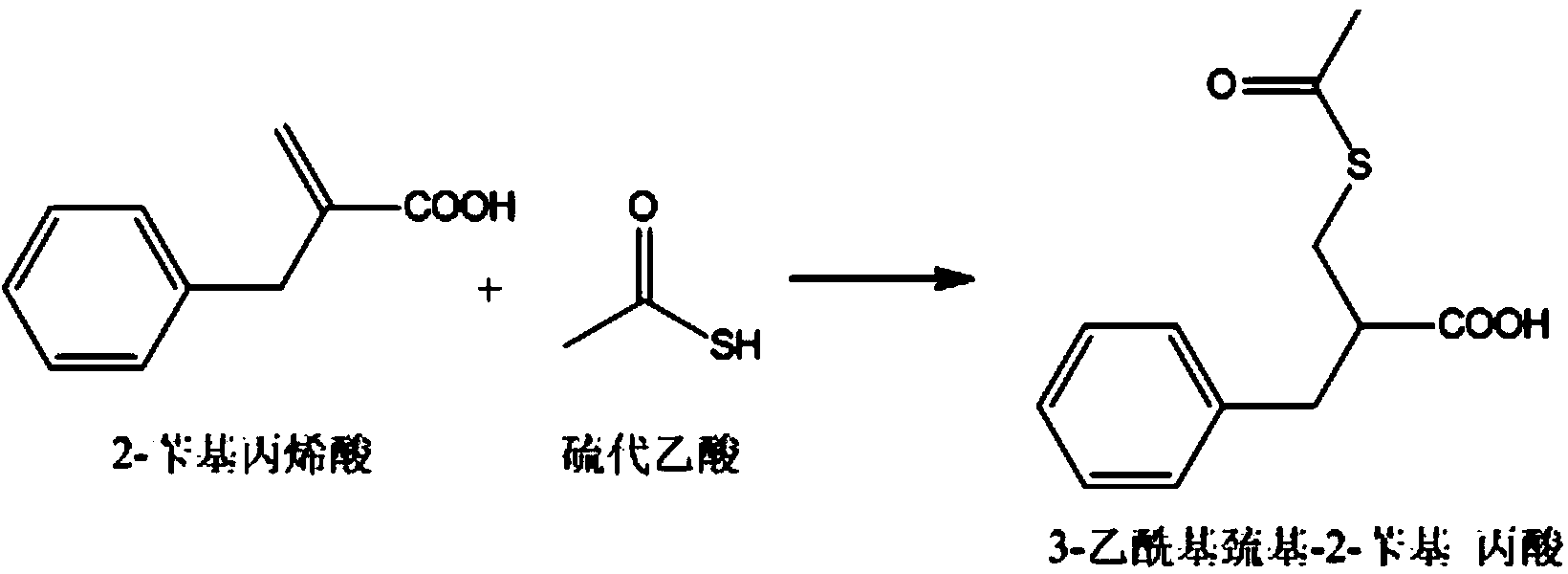
[0046] 1] Preparation of intermediate 3-acetylmercapto-2-benzylpropionic acid
[0047] 1.1] Add 26.75g of 2-benzylacrylic acid to a 250ml reaction flask, then add 20.06g of thioacetic acid, stir to dissolve; slowly raise the temperature to 30°C; keep it warm for 8 hours, and generate the intermediate 3-acetylmercapto-2-benzyl A solution of propionic acid;
[0048] 1.2] After the reaction in step 1.1 is completed, distill under reduced pressure. The end point temperature of the distillation is about 78°C (75~80°C), and the vacuum degree is ≥-0.08MPa; Distill toluene, the distillation end point temperature is 78°C (75-80°C), and vacuum dry; after distillation, take a sample and check the purity of the liquid phase, the purity of the intermediate 3-acetylmercapto-2-benzylpropionic acid is ≥95.0%, thio Acetic acid≤0.1%.
[0049] In this step, thioacetic acid is both a reagent and a solvent, and the thioacetic acid is distilled off after the reaction of this step is completed.
...
Embodiment 2
[0059] 1] Preparation of intermediate 3-acetylmercapto-2-benzylpropionic acid
[0060] 1.1] Add 26.75g of 2-benzylacrylic acid to a 250ml reaction bottle, then add 20.06g of thioacetic acid, stir to dissolve; slowly raise the temperature to 60°C; keep it warm for 5 hours;
[0061] 1.2] After the reaction in step 1.1 is completed, distill under reduced pressure. The end point temperature of the distillation is about 78°C (75~80°C), and the vacuum degree is ≥-0.08MPa; Distill toluene, the distillation end point temperature is 78°C (75-80°C), and vacuum dry; after distillation, take a sample and check the purity of the liquid phase, the purity of the intermediate 3-acetylmercapto-2-benzylpropionic acid is ≥95.0%, thio Acetic acid≤0.1%.
[0062] In this step, thioacetic acid is both a reagent and a solvent, and the thioacetic acid is distilled off after the reaction.
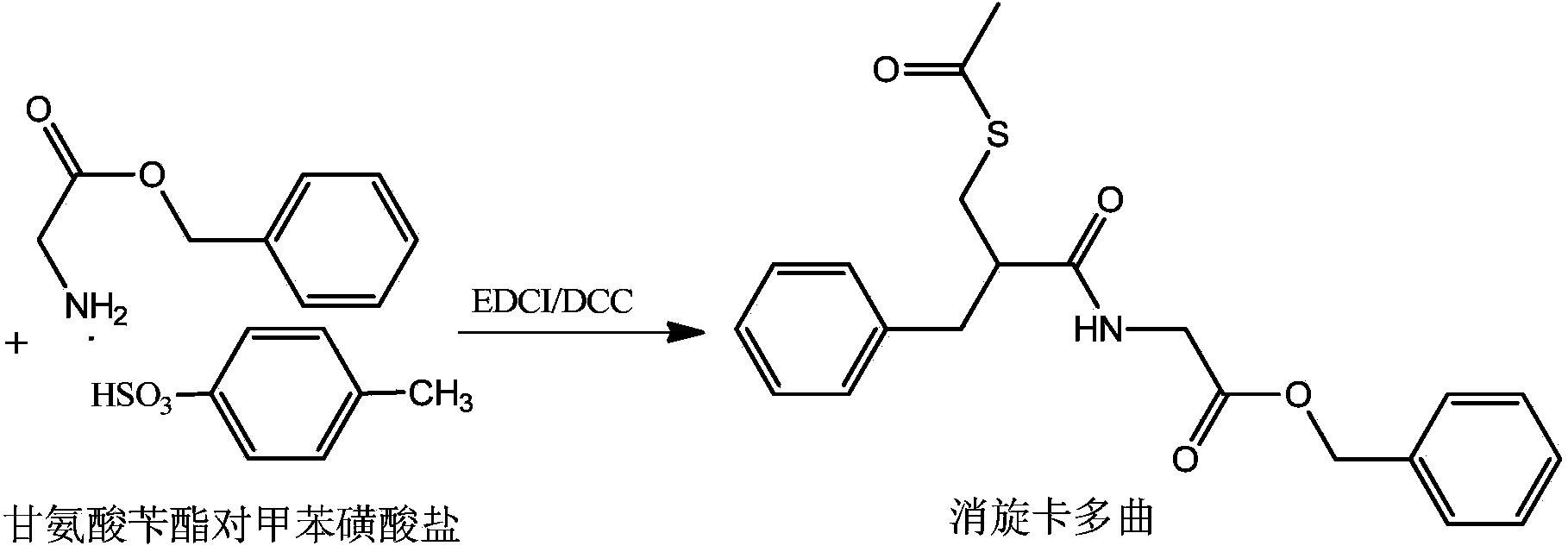
[0063] 2) Preparation of racecadotril
[0064] 2.1] Put 55.65g of glycine benzyl ester p-toluenesulfonate into...
Embodiment 3
[0070] 1) Preparation of 3-acetylmercapto-2-benzylpropionic acid
[0071] 1.1] Add 26.75g of 2-benzylacrylic acid to a 250ml reaction flask, then add 25.11g of thioacetic acid, stir to dissolve; slowly raise the temperature to 90°C; keep the reaction for 2 hours.
[0072] 1.2] After the reaction in step 1.1 is completed, distill under reduced pressure. The end point temperature of the distillation is about 78°C (75~80°C), and the vacuum degree is ≥-0.08MPa; Distill toluene, the distillation end point temperature is 78°C (75-80°C), and vacuum dry; after distillation, take a sample and test the purity of the liquid phase, the purity is ≥95.0%, and the thioacetic acid is ≤0.1%.
[0073] 2) Preparation of racecadotril
[0074] 2.1] Put 55.65g of glycine benzyl ester p-toluenesulfonate in another reaction flask and dissolve it with 165ml of dichloromethane, add 26.3g of pyridine; stir to dissolve; cool the system to 2°C (-2~5°C), add 3- Acetylmercapto-2-benzylpropionic acid solut...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com