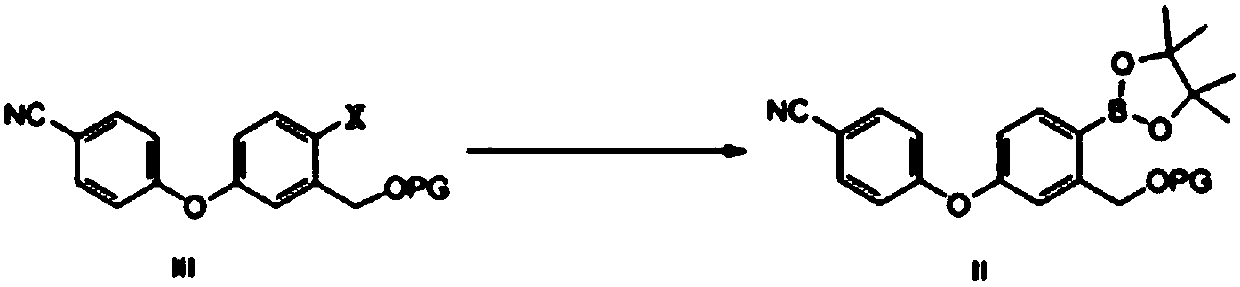
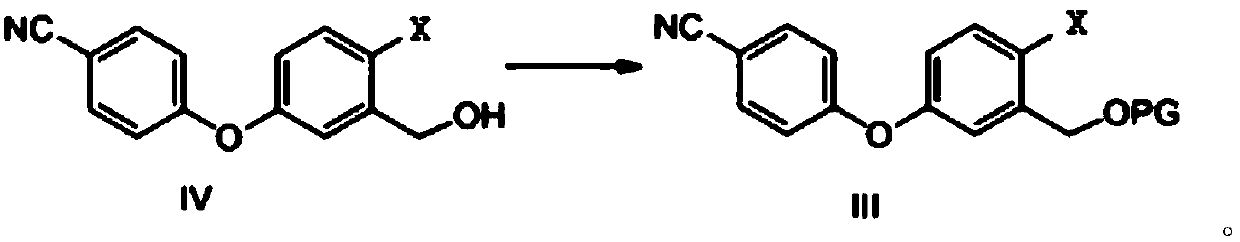
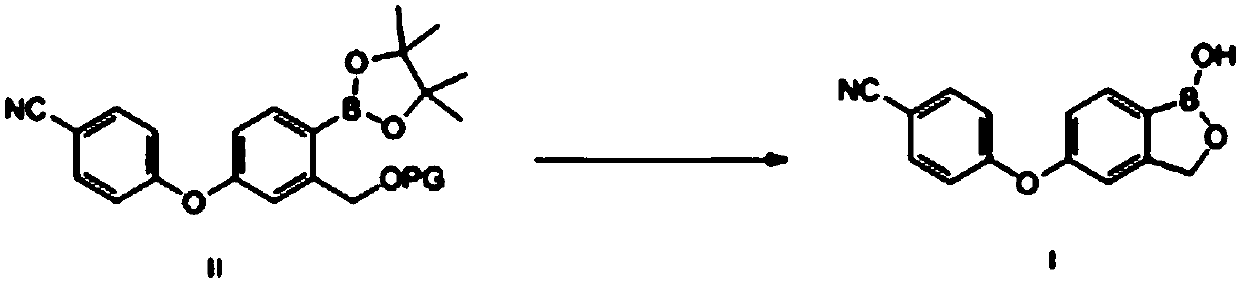Method for preparing crisaborole
A technology of crisaborol and compounds, which is applied in the field of preparation of crisaborol, can solve the problems of high cost and low yield, and achieve the effects of low cost, high reaction yield and easier quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1 Formula III compound (wherein X is bromine, PG is the synthesis of acetyl group)
[0063] Compound IV (30.00g, 98.7mmol, 1.0eq), acetic anhydride (12.08g, 118.4mmol, 1.2eq), THF (150mL) and DMAP (15.65g, 128.3mmol, 1.3eq) were added to the reaction flask respectively, The reaction was stirred at room temperature. After 1 h, TLC showed that the reaction of the raw materials was basically completed, and the post-treatment was carried out. Add 200mL of water and 200mLEA, separate the layers, wash the organic phase with water, dry, and concentrate to obtain a total of 32.36g of off-white solid, which is the target compound of formula III, namely 2-bromo-5-(4-cyanophenoxy)benzyl alcohol acetic acid Esters, yield 95%.
[0064] The nuclear magnetic data of gained white solid is as follows:
[0065] 1 H-NMR (400Hz, CDCl 3 ) δ (ppm) 2.15 (s, 3H), 5.18 (s, 2H), 6.92 (dd, J = 4Hz, 8Hz, 1H), 7.04 (m, 2H), 7.14 (d, J = 4Hz, 1H), 7.59 (d, J=8Hz, 1H), 7.64 (m, 2H). ...
Embodiment 2
[0066] Example 2 Synthesis of the compound of formula III (wherein X is bromine, and PG is 2,3,4-trimethoxybenzyl)
[0067] Dissolve compound IV (1.00g, 3.3mmol, 1.0eq) in DMF (15mL), add 60% sodium hydride (0.16g, 4.0mmol, 1.2eq), stir at room temperature for 30min, add 2,3,4-trimethyl Oxybenzyl chloride (1.00g, 4.6mmol, 1.4eq), heated to 85°C and stirred for reaction. After 6h, TLC showed that the reaction of the raw materials was basically completed, and the post-treatment was carried out. The reaction solution was cooled to room temperature, 20 mL of water was added to quench the reaction, extracted with 50 mL of EA, separated, the organic phase was washed with water, dried and concentrated, and the obtained crude product was purified by column chromatography to obtain 1.46 g of yellow oil, which was the target compound, with a yield of 76 %.
Embodiment 3
[0068] Synthesis of Example 3 Compound of Formula III (wherein X is bromine, and PG is trimethylsilyl)
[0069] Compound IV (3.00g, 9.9mmol, 1.0eq) and imidazole (1.34g, 19.7mmol, 2.0eq) were dissolved in dichloromethane (25mL), cooled to -15°C, and trimethylchlorosilane (1.60g, 14.7mmol, 1.5eq), the reaction was stirred. After 16h, TLC showed that the basic reaction of the raw materials was completed, and the post-treatment was carried out. The reaction was quenched by adding 50 mL of water, extracted with 50 mL of dichloromethane, separated, the organic phase was washed with water, dried, and concentrated. The obtained crude product was purified by column chromatography to obtain 3.26 g of a yellow oil, which was the target compound, with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com