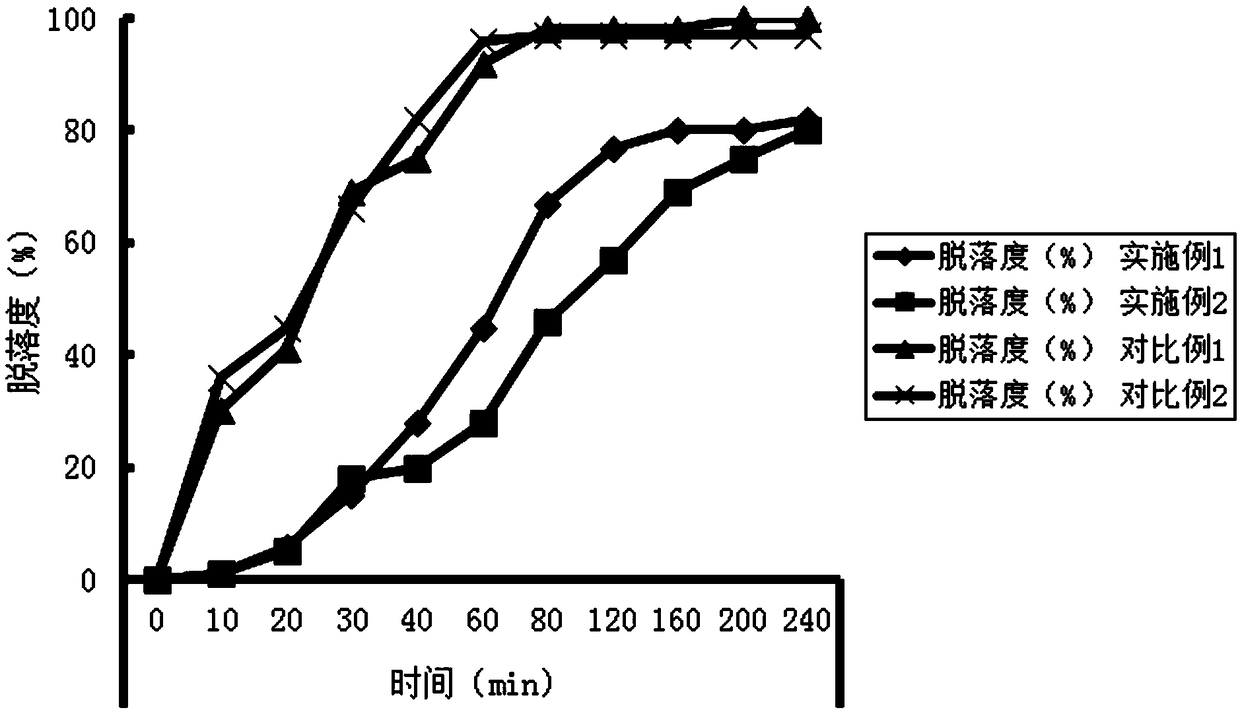
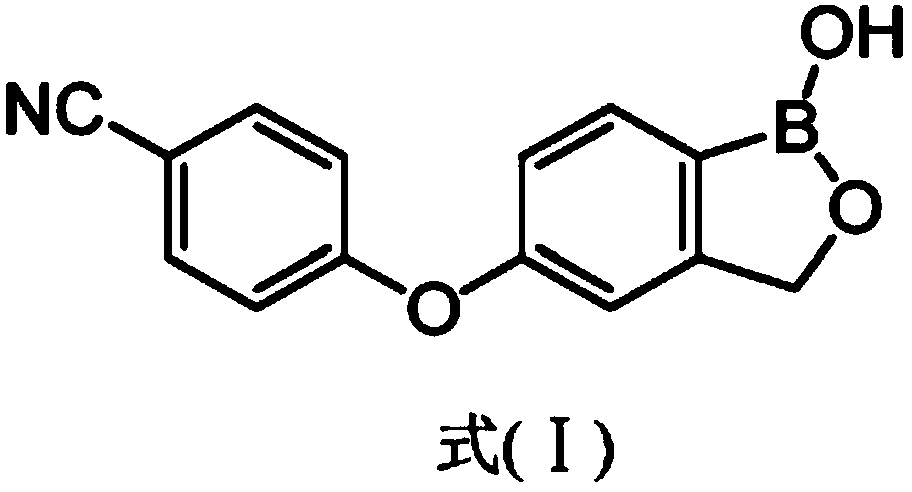
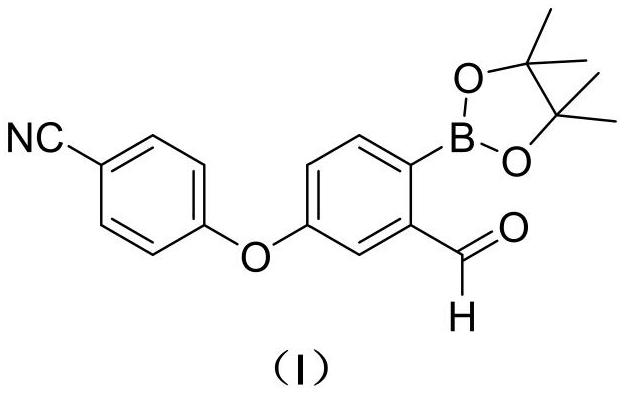
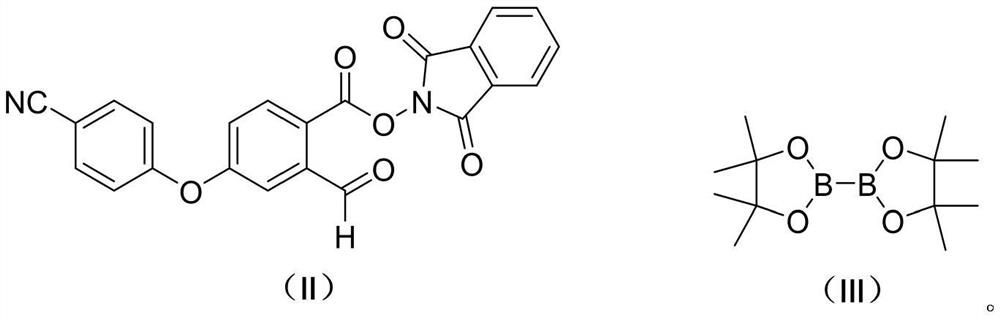
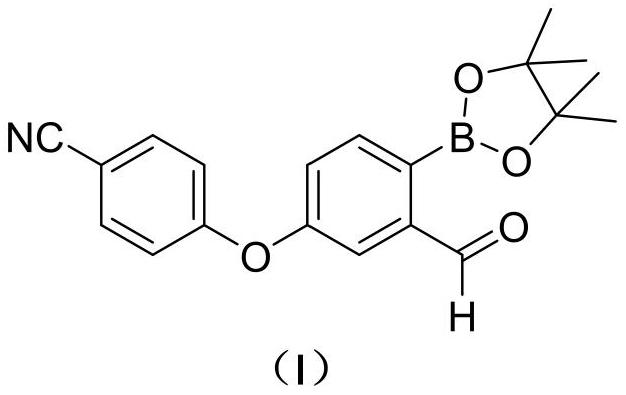
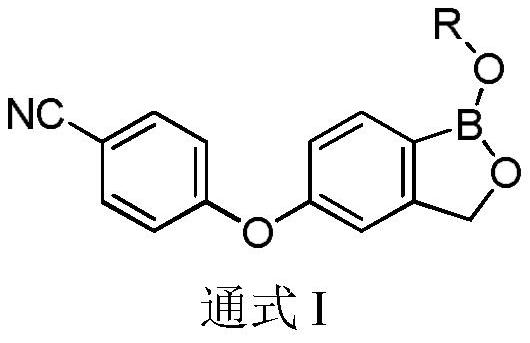
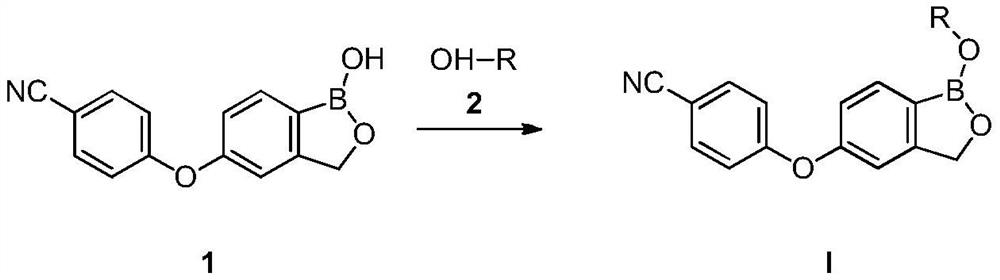
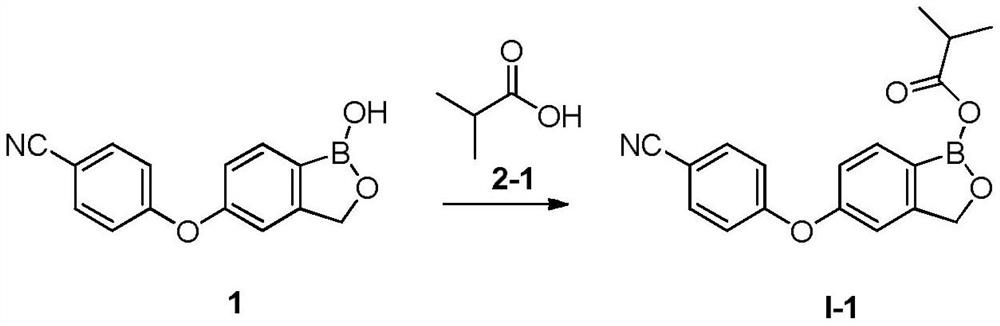
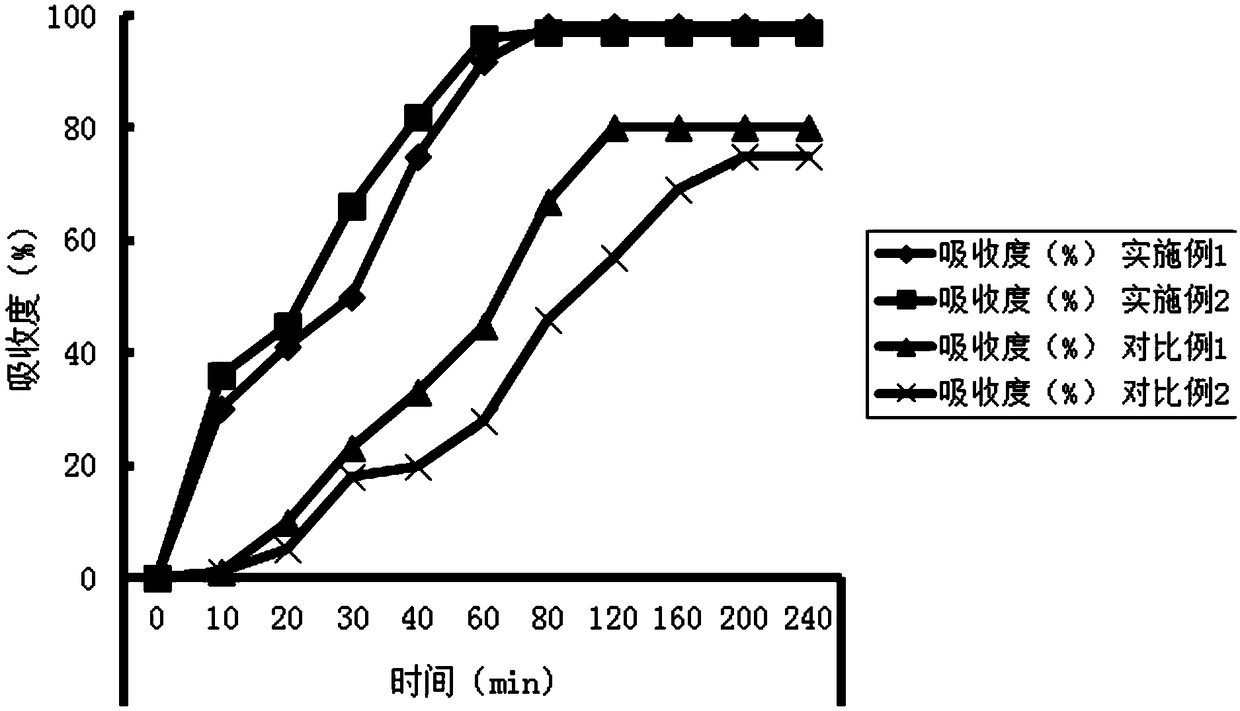
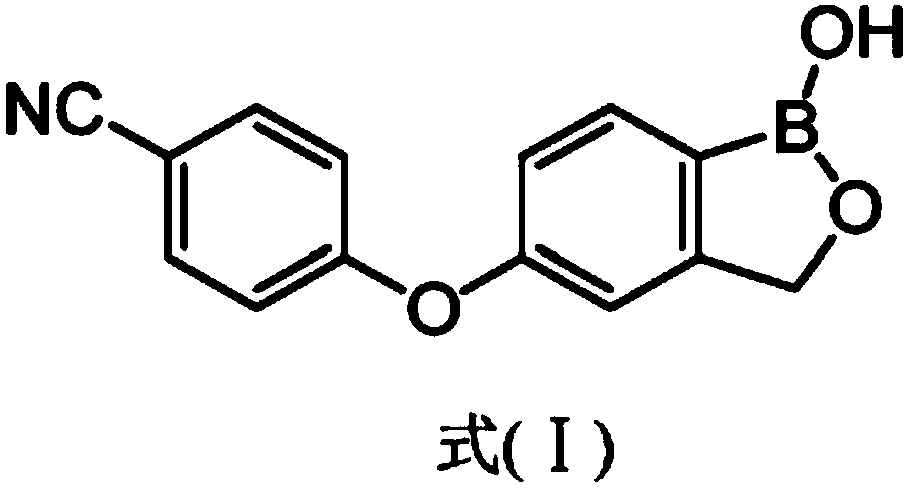
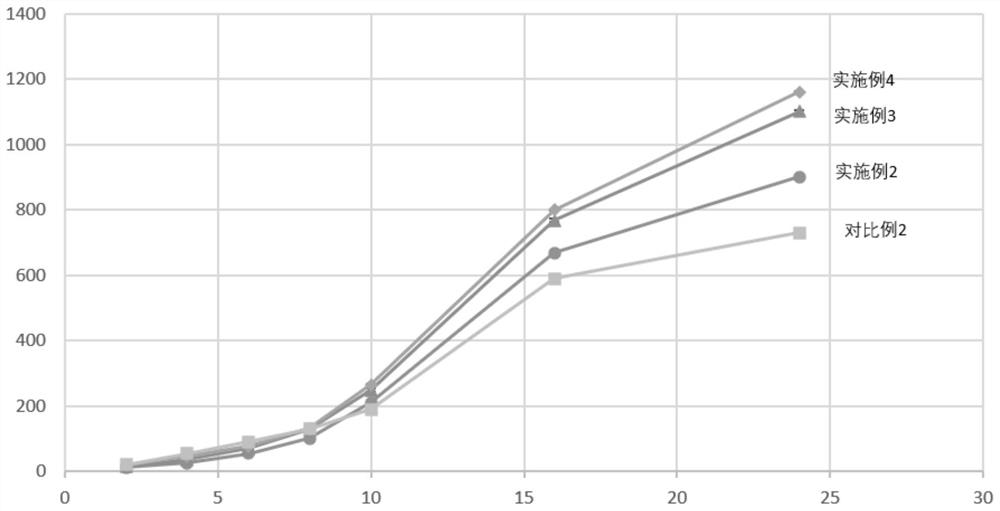
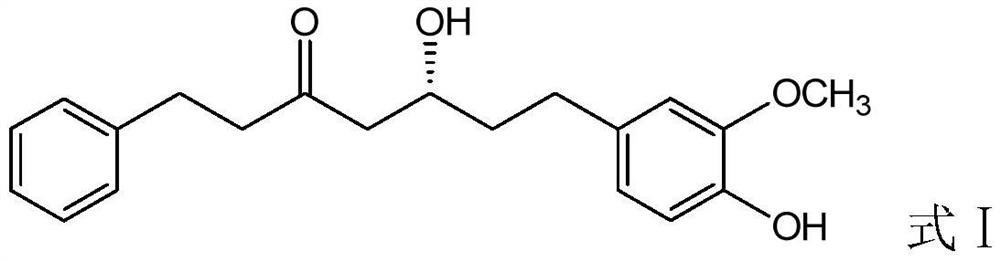
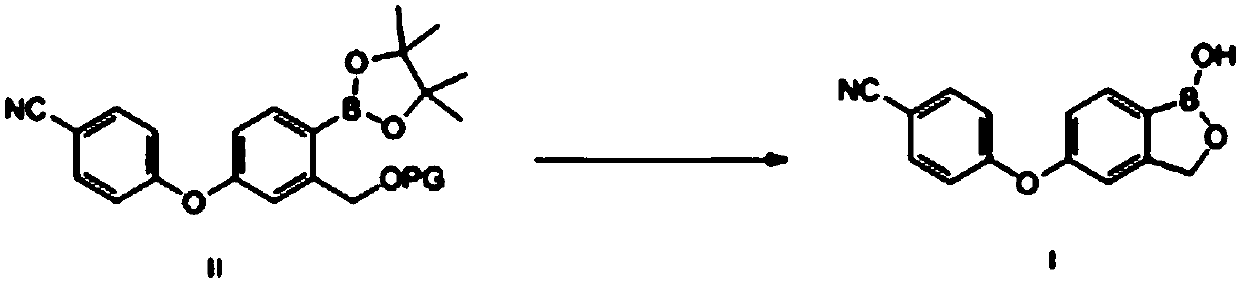Patents
Literature
41 results about "Crisaborole" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
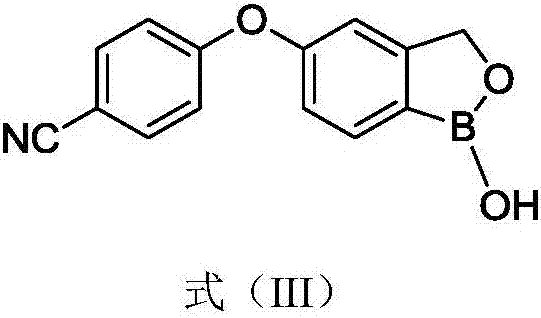
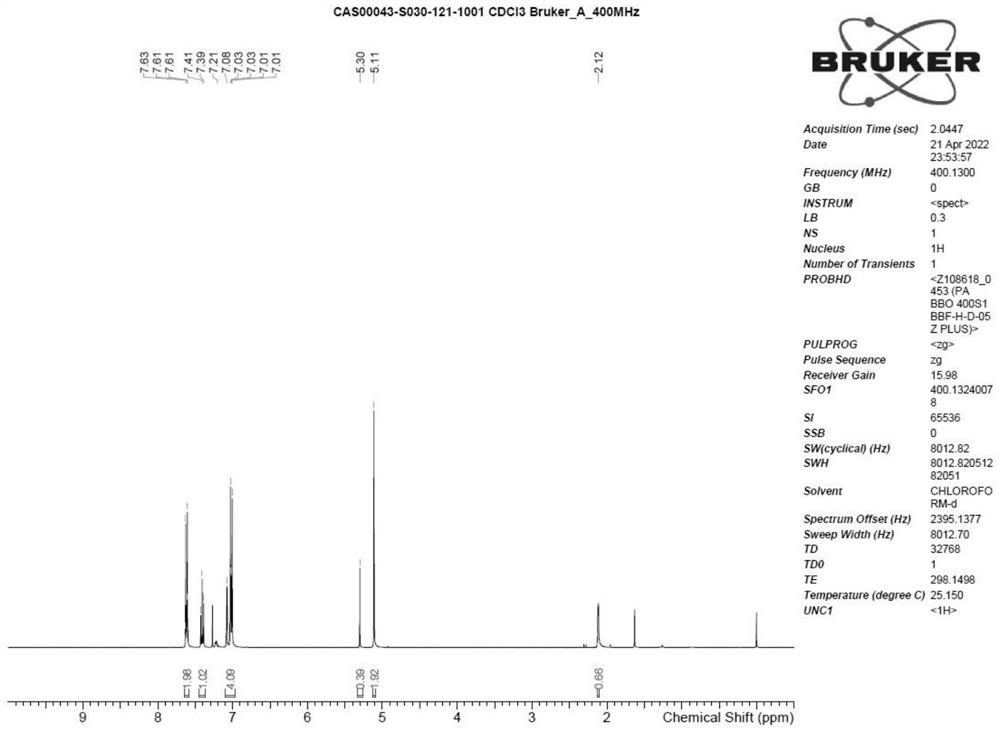
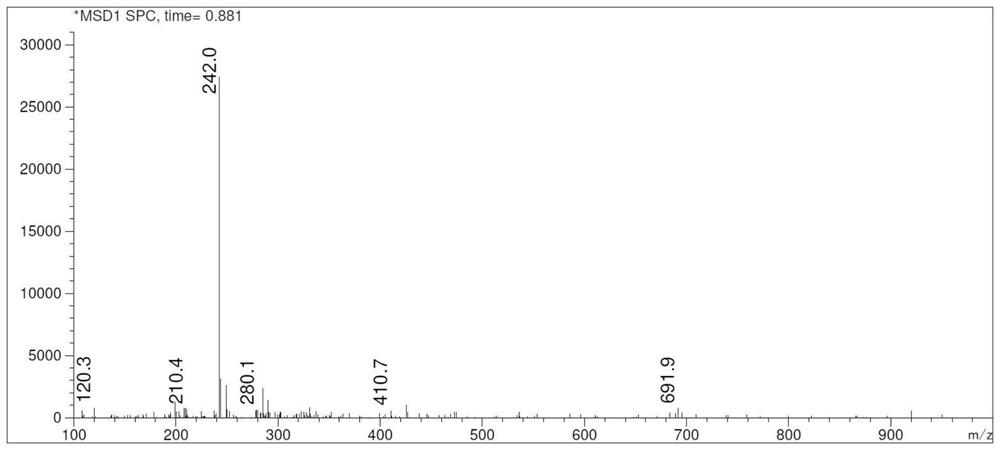
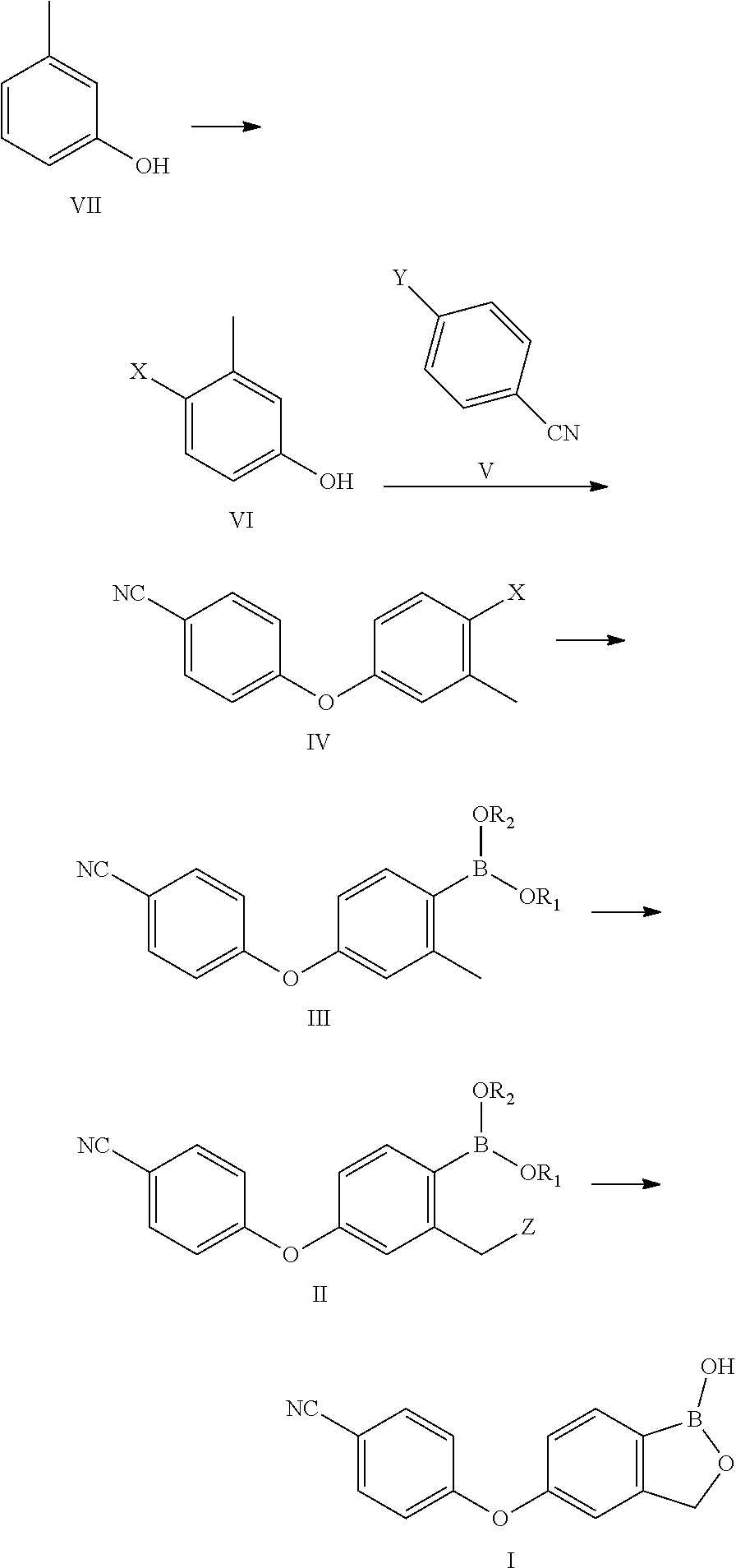
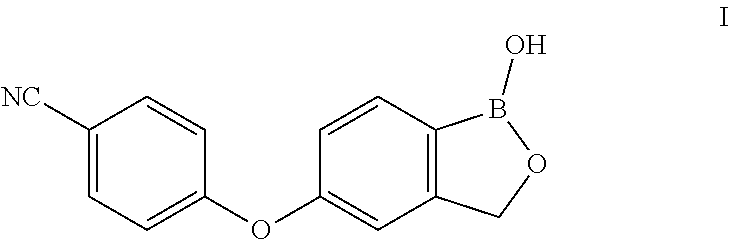
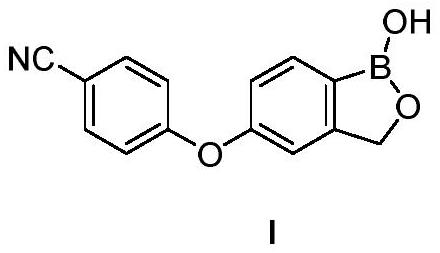
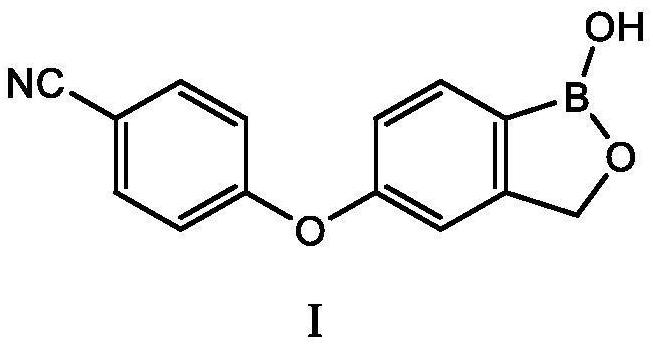
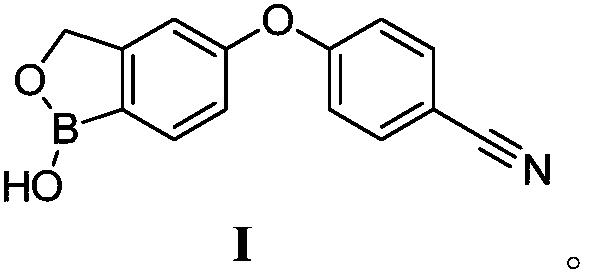
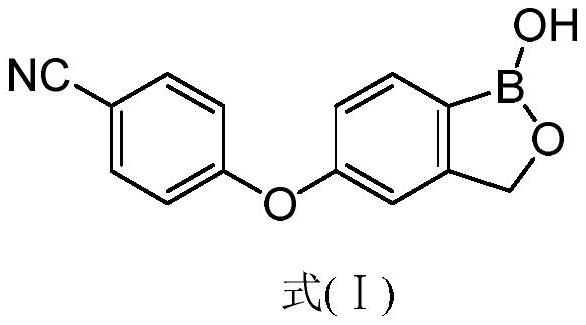
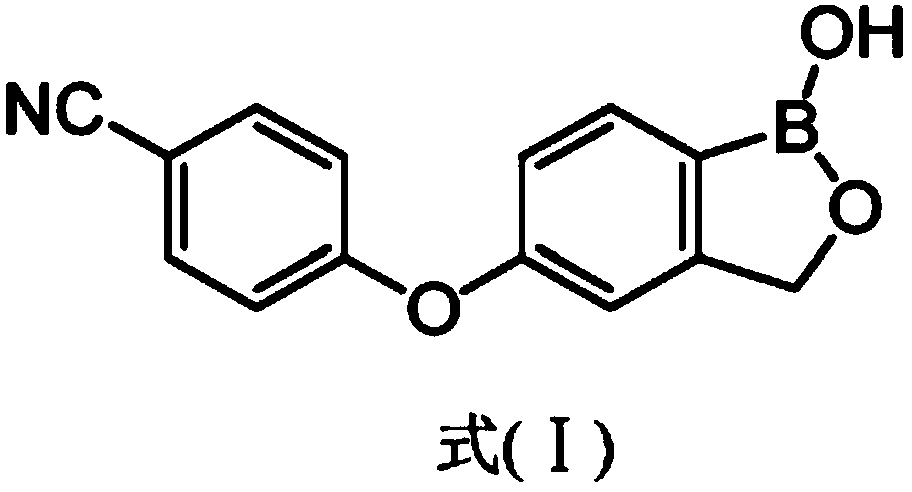
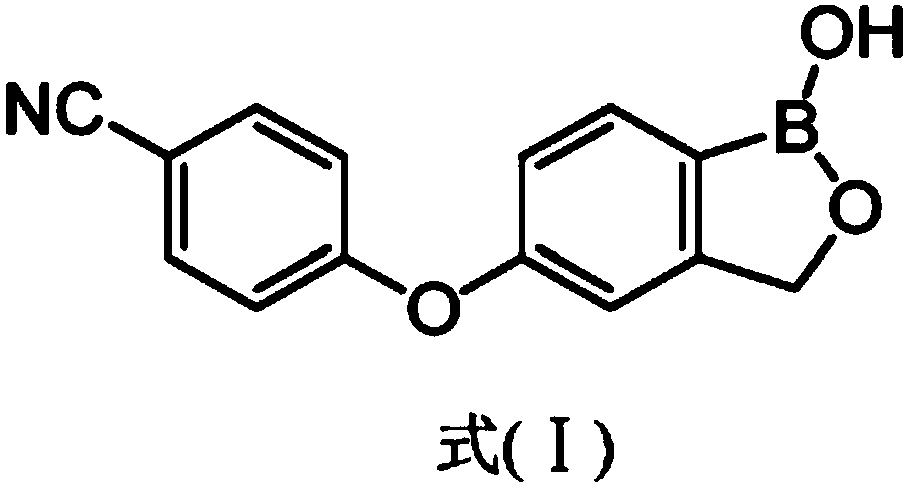
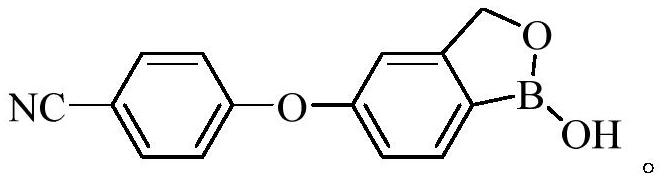
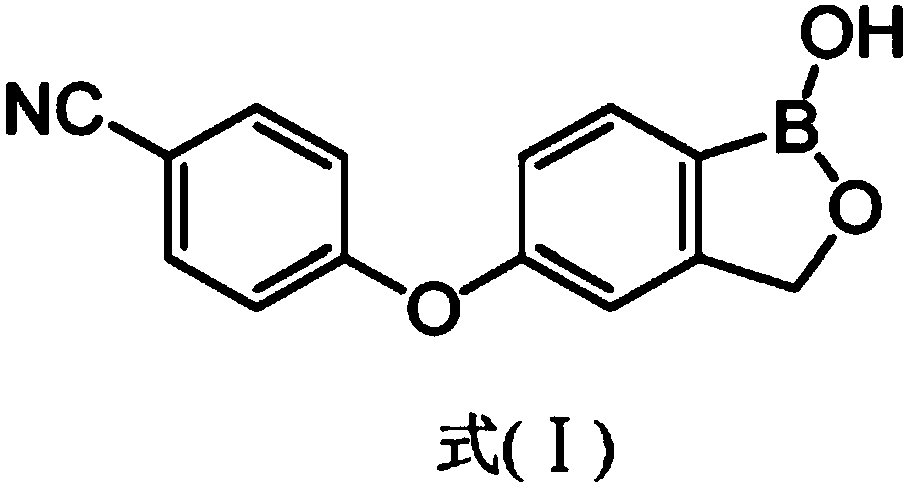
Crisaborole (trade name Eucrisa) is a nonsteroidal topical medication used for the treatment of mild-to-moderate atopic dermatitis (eczema) in people two years or older. It was approved by U.S. Food and Drug Administration on Dec 14, 2016 and June 6, 2018 by Health Canada.
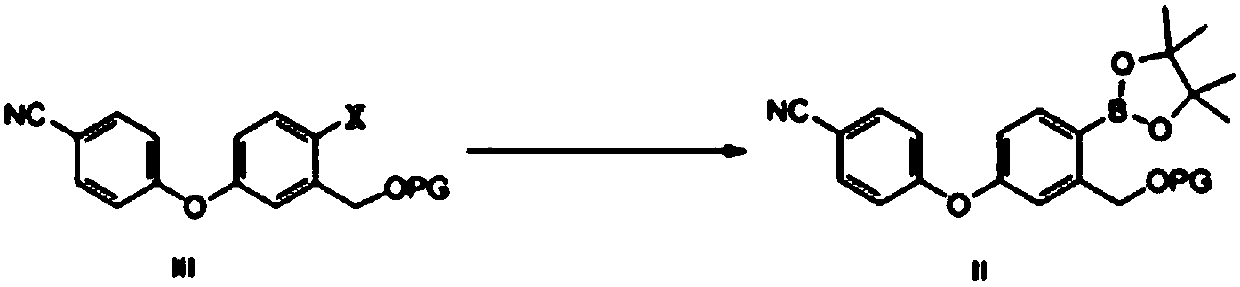
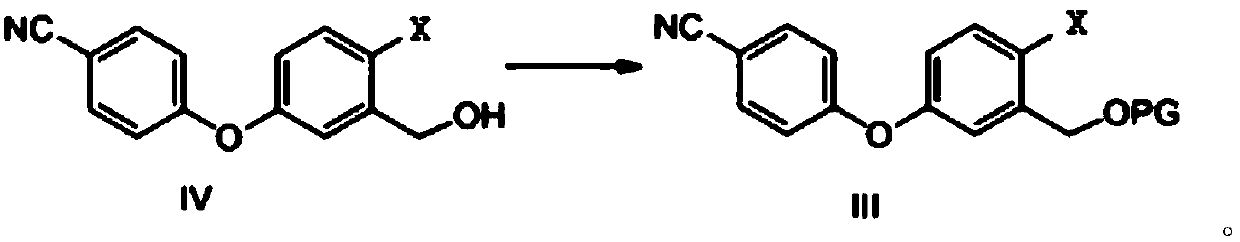
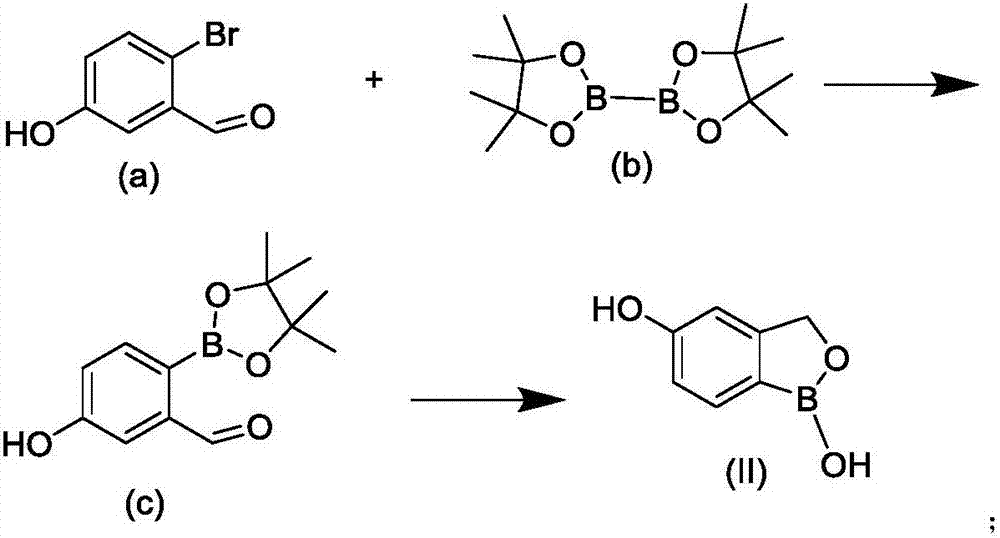
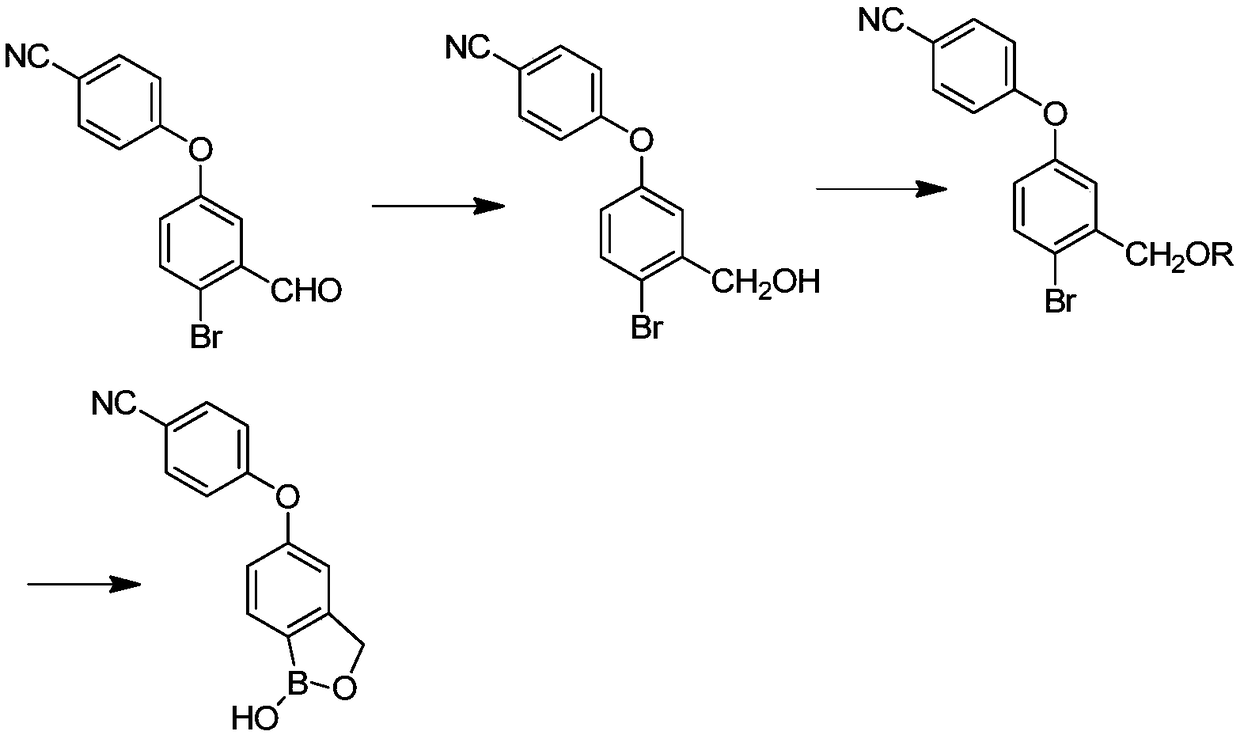
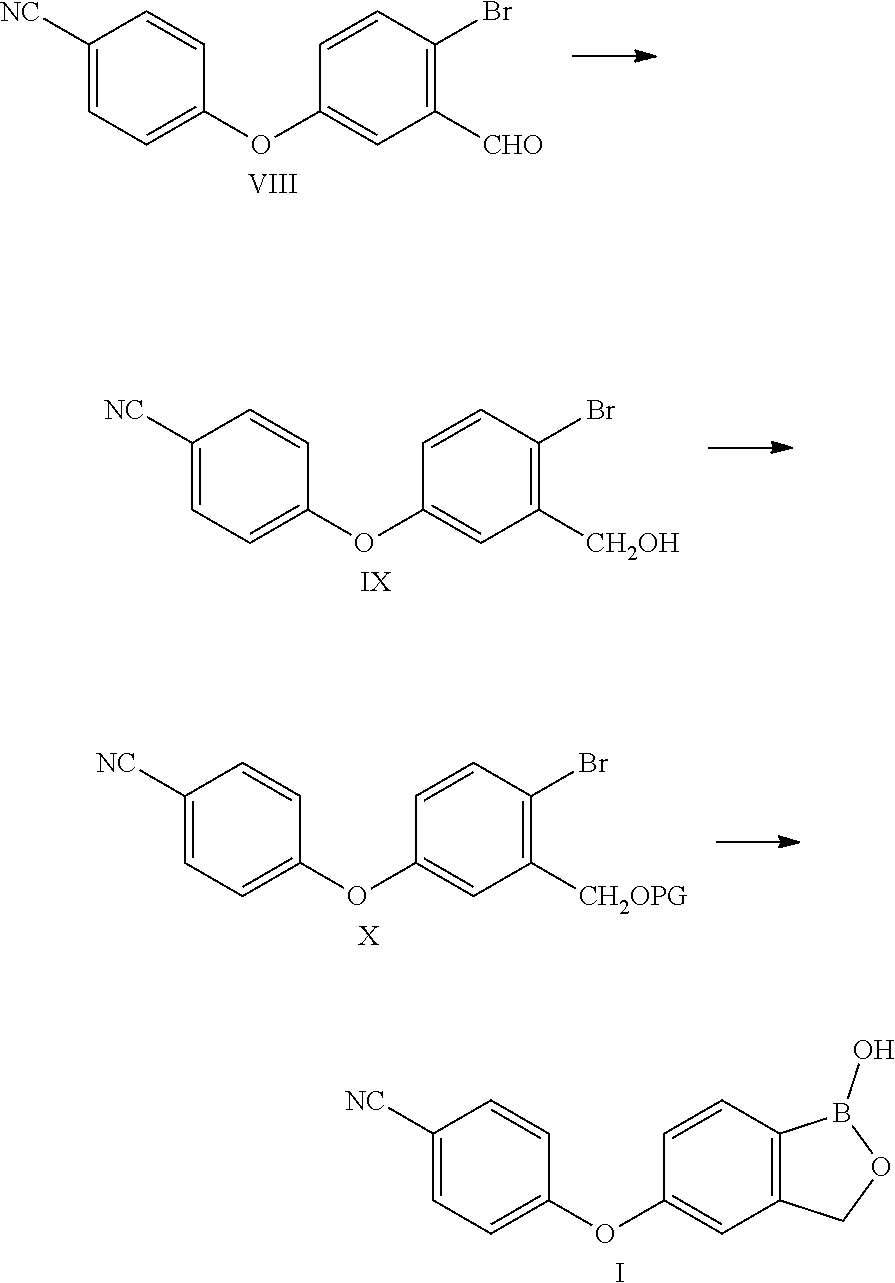
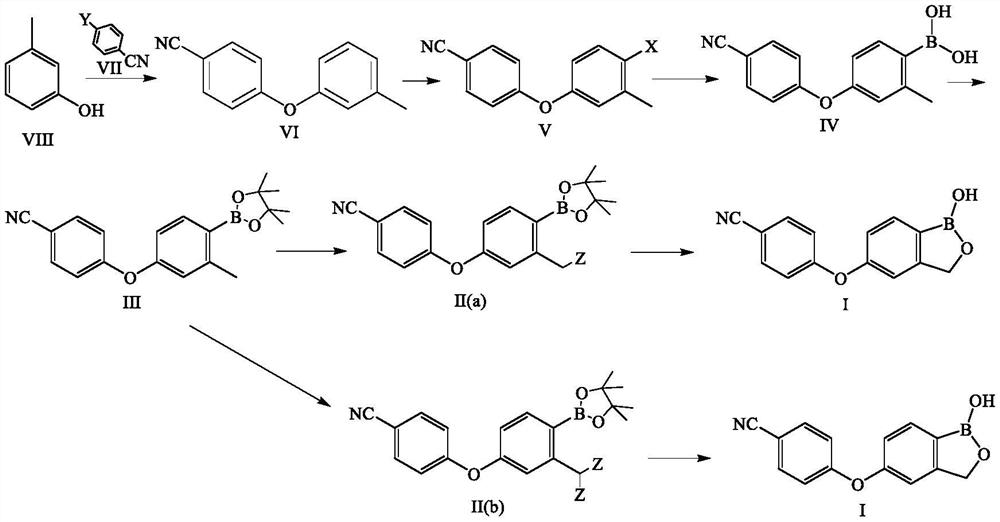
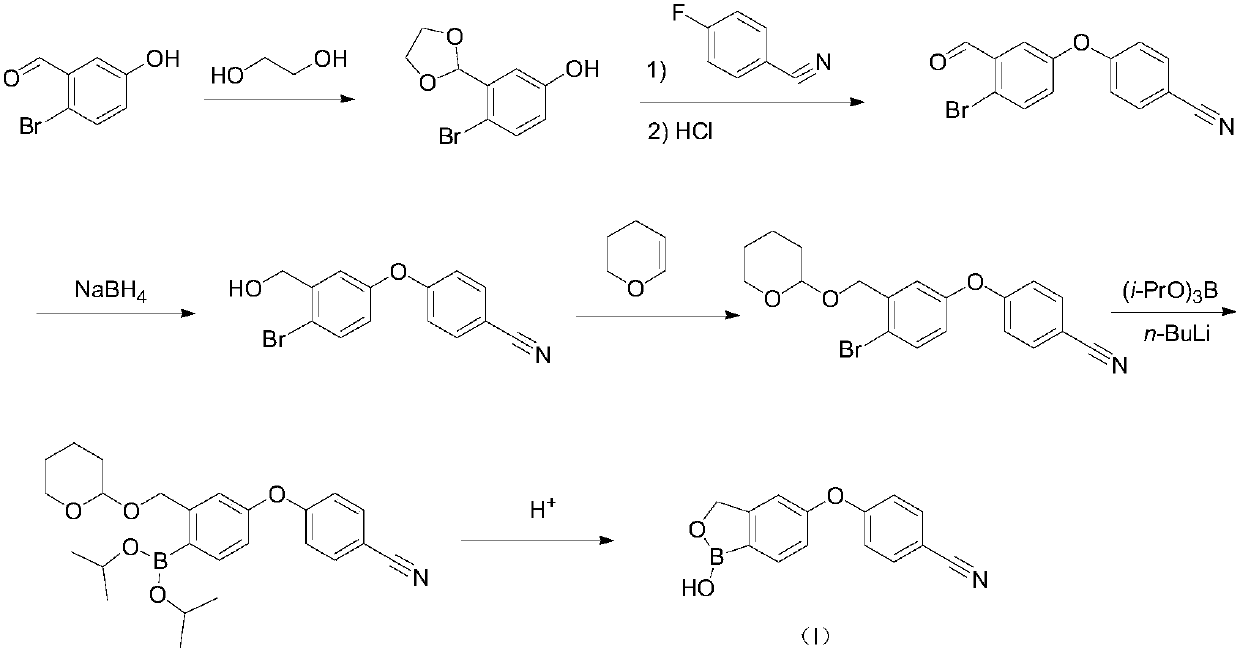
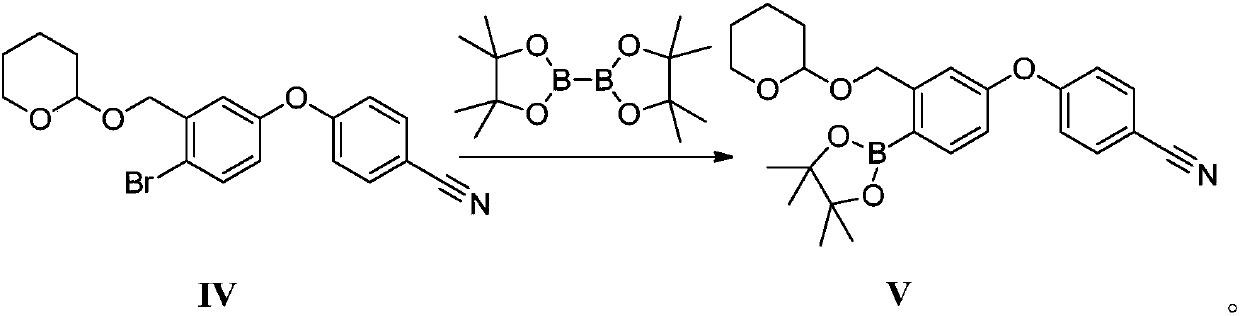
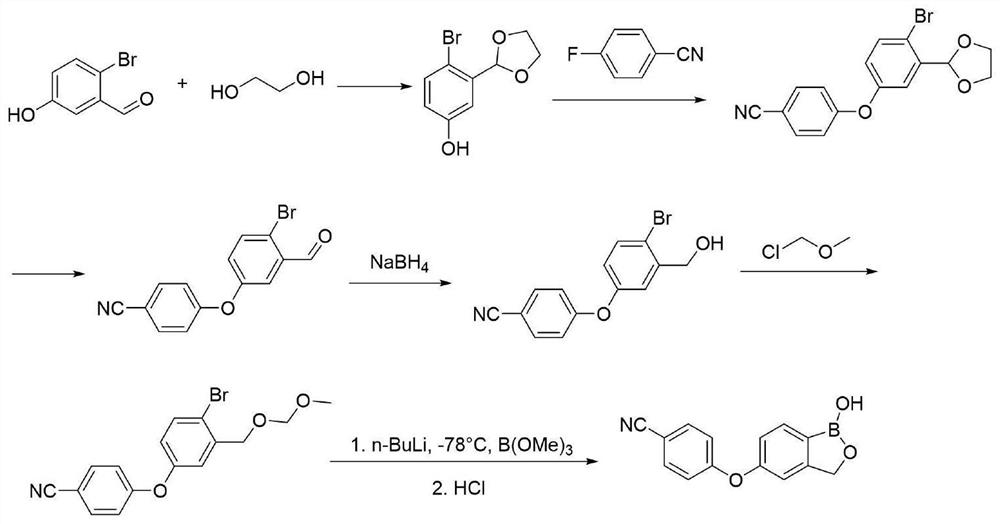
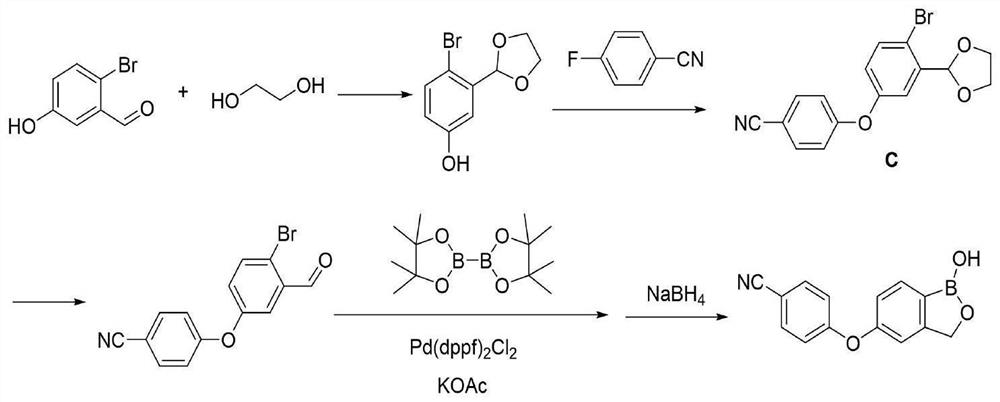
Method for preparing crisaborole
ActiveCN108047261AMild reaction conditionsEasy to operateGroup 3/13 element organic compoundsBulk chemical productionPhenylboronic acidOrganic solvent
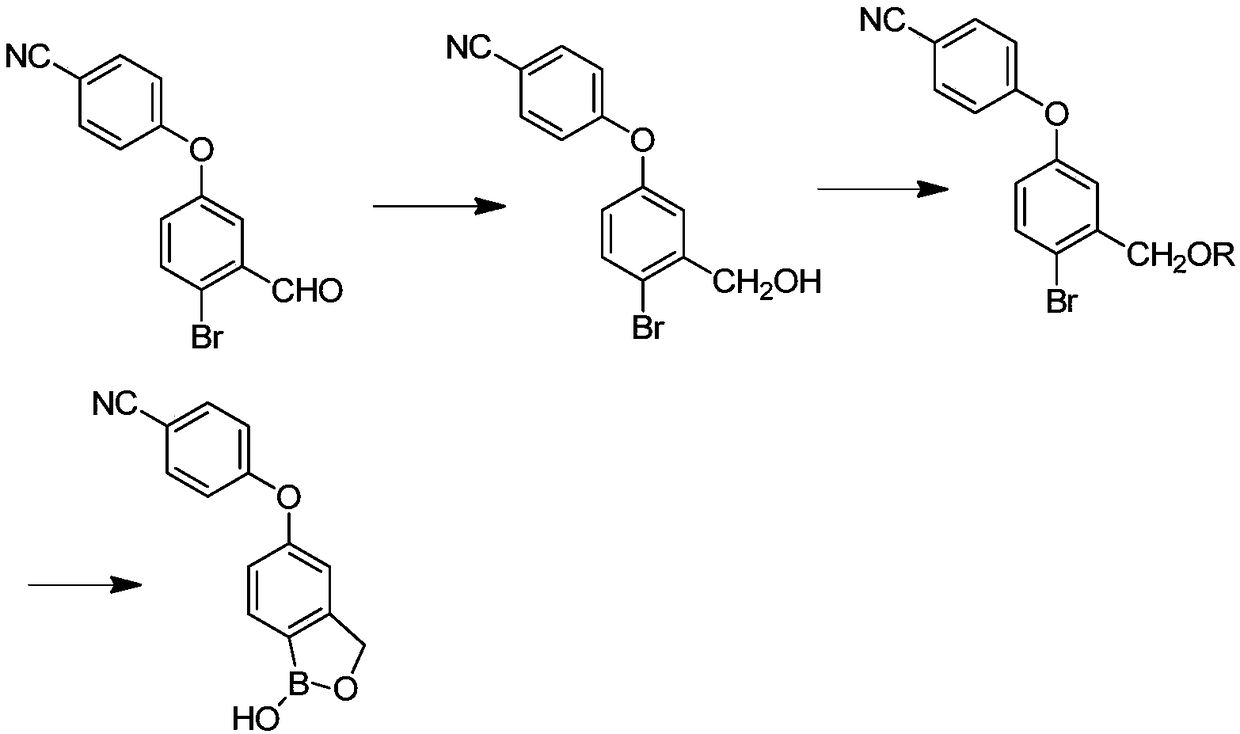
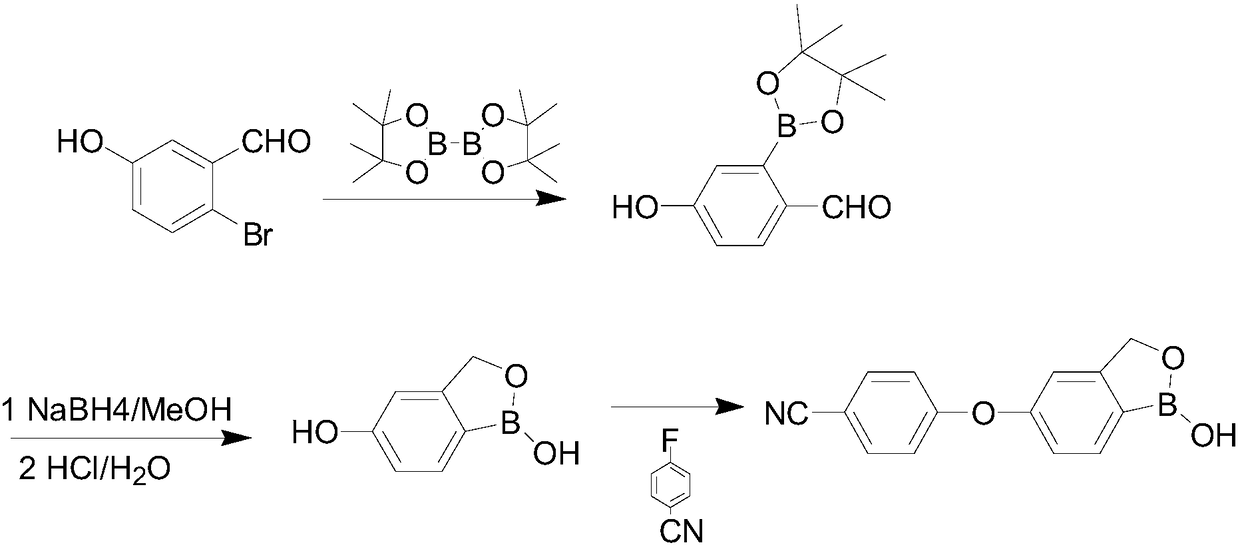
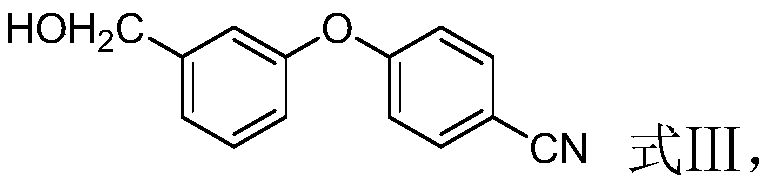
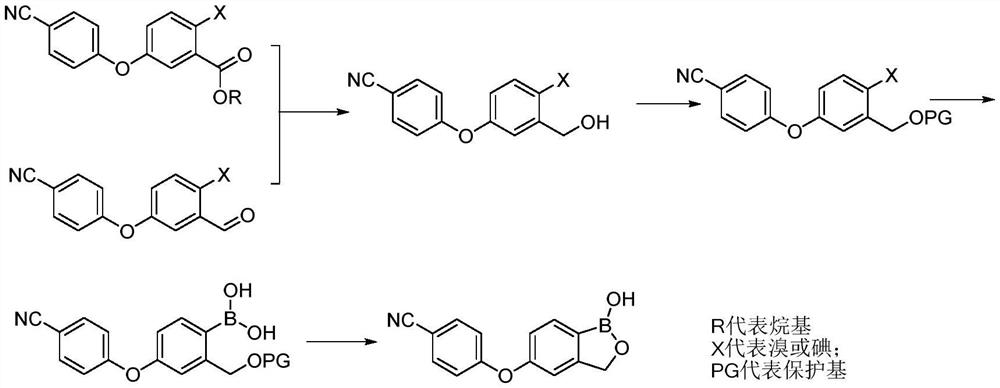
The invention discloses a method for preparing crisaborole and an intermediate for preparing crisaborole. The method comprises the following steps: (1) enabling components such as 2-halogen-5-(4-cyanphenoxy) benzyl acetate and bis(pinacolato)diboron to react in the presence of an organic solvent and a palladium catalyst under an alkali condition so as to obtain components such as 2-acetoxyl methyl-4-(4-cyan phenoxy) phenylboronic acid pinacol ester; (2) enabling components such as the 2-acetoxyl methyl-4-(4-cyan phenoxy) phenylboronic acid pinacol ester to react in a solvent under an acid oralkali condition, thereby obtaining crisaborole. The method is gentle in reaction condition, easy to operate, high in reaction yield, simple in aftertreatment, relatively low in cost and applicable toindustrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD +1
Synthesis method of crisaborole
InactiveCN106928264AHigh yieldHigh purityGroup 3/13 element organic compoundsOrganic solventSynthesis methods
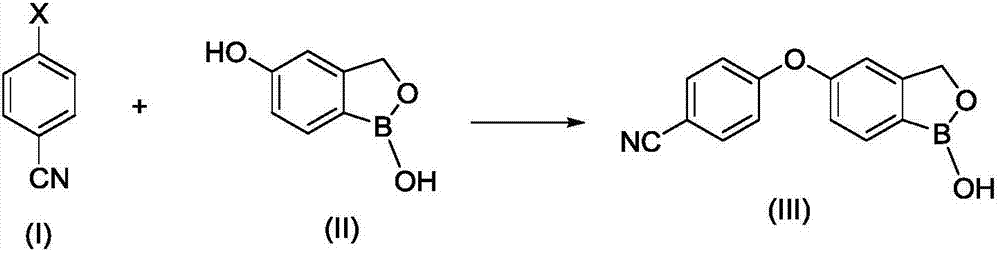
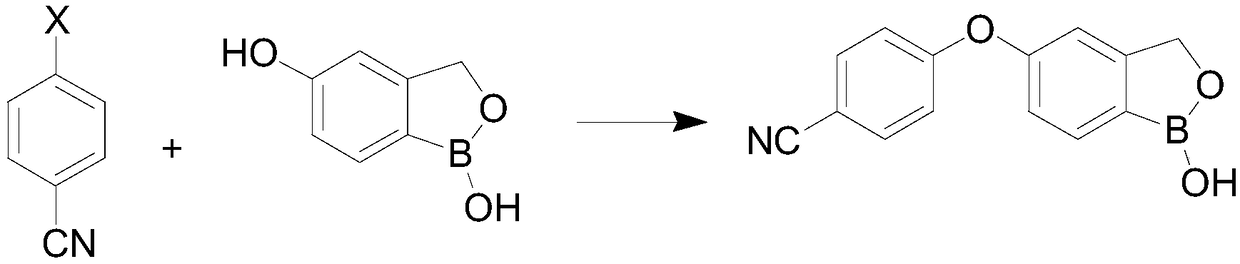
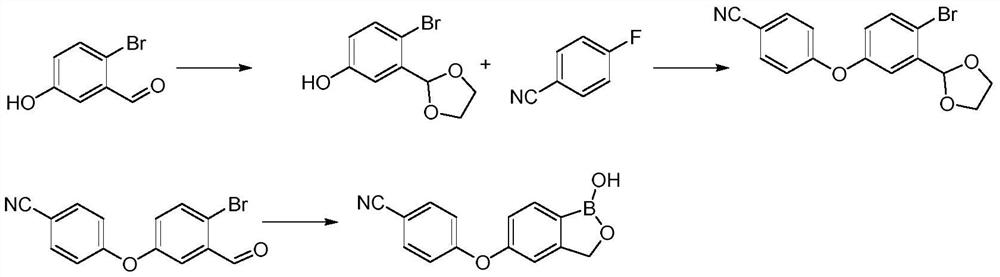
The invention provides a synthesis method of crisaborole, belongs to the technical field of drug synthesis, and relates to a synthesis method of a novel boron-containing anti-inflammatory preparation, namely crisaborole. 4-halobenzonitrile and benzo[c][1,2] oxaborolane-1,5(3H)-diol are reacted in an organic solvent in the presence of alkali, and the crisaborole is obtained. The synthesis methoid is simple, and the crisaborole is high in yield and high in purity. (The reaction formula is shown in the description).
Owner:湖南中智优库科技有限公司
Preparation method of crisaborole
InactiveCN108659024ALow priceMild reaction conditionsGroup 3/13 element organic compoundsTert-butyldimethylsilyl chlorideTrimethyl borate
The invention discloses a preparation method of crisaborole. The method comprises the steps as follows: a compound shown in a formula I and alkali metal borohydride are subjected to a contact reaction, and a compound shown in a formula II is obtained; the compound shown in the formula II and a compound a are subjected to a contact reaction, and a compound III is obtained; or the compound shown inthe formula II and dihydropyran are subjected to a contact reaction, and a compound III is obtained, wherein the compound a is trimethylchlorosilane, tert-butyldimethylsilyl chloride or chloromethyl methyl ether; the compound shown in the formula III and an isopropylmagnesium chloride solution are subjected to a contact reaction, and a standby solution is obtained; then, the standby solution is added to a mixed solution of a compound b and a third organic solvent for a contact reaction, hydrochloric acid is added for a contact reaction, and crisaborole, namely, a compound shown in a formula IV, is obtained, wherein the compound b is 2-alkoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane, triisopropyl borate or trimethyl borate.
Owner:WUHAN POLYTECHNIC UNIVERSITY
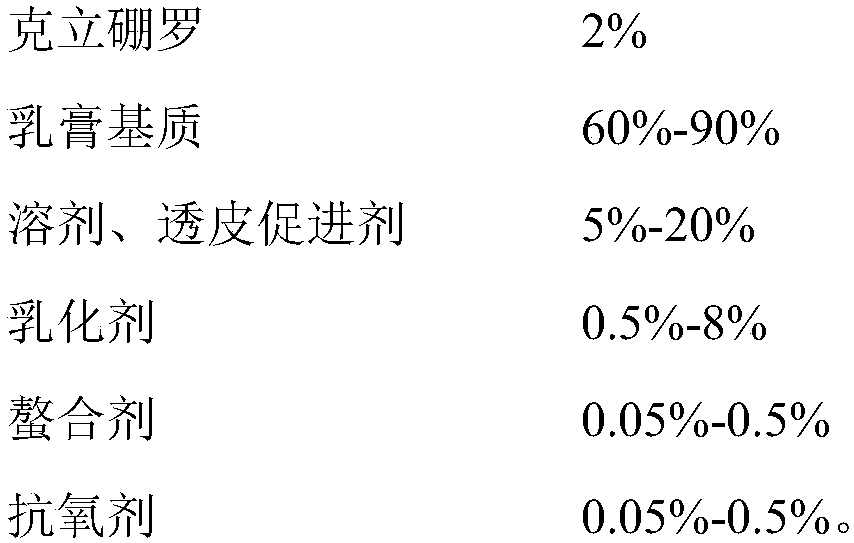
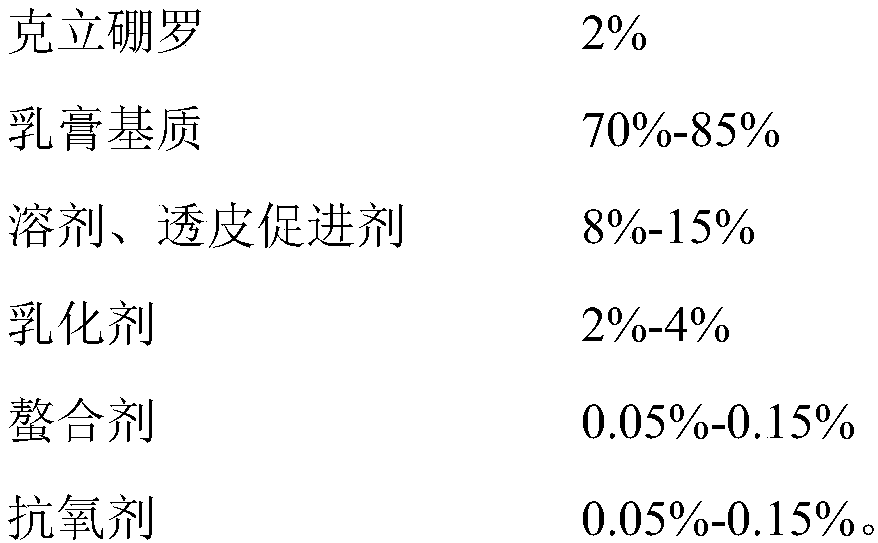
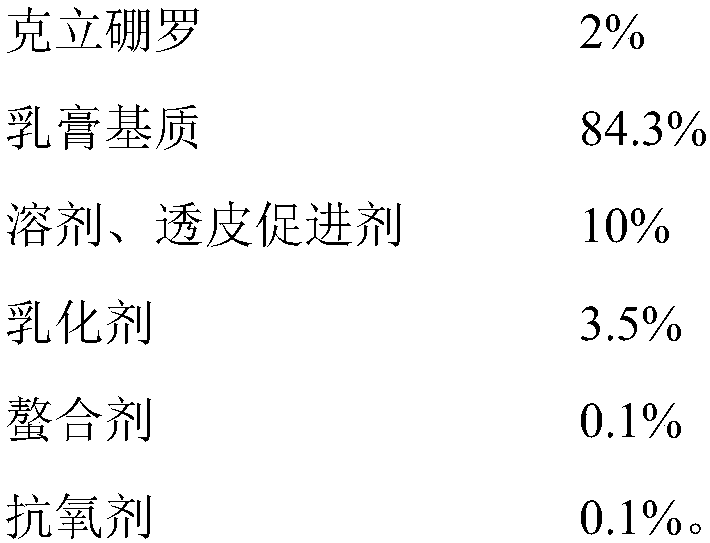
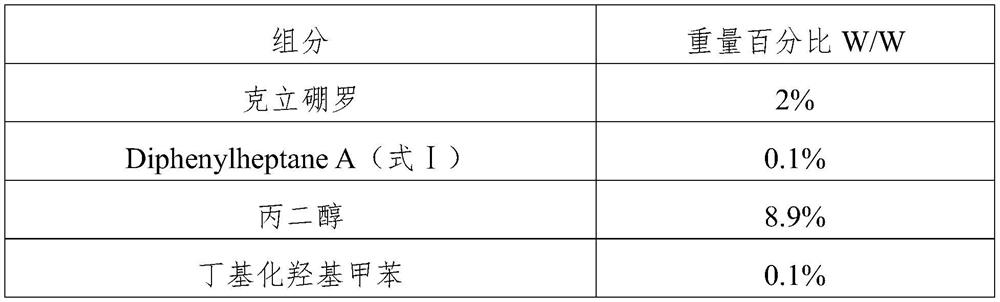
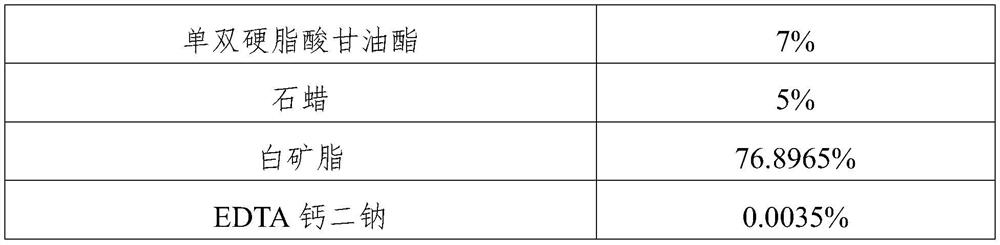
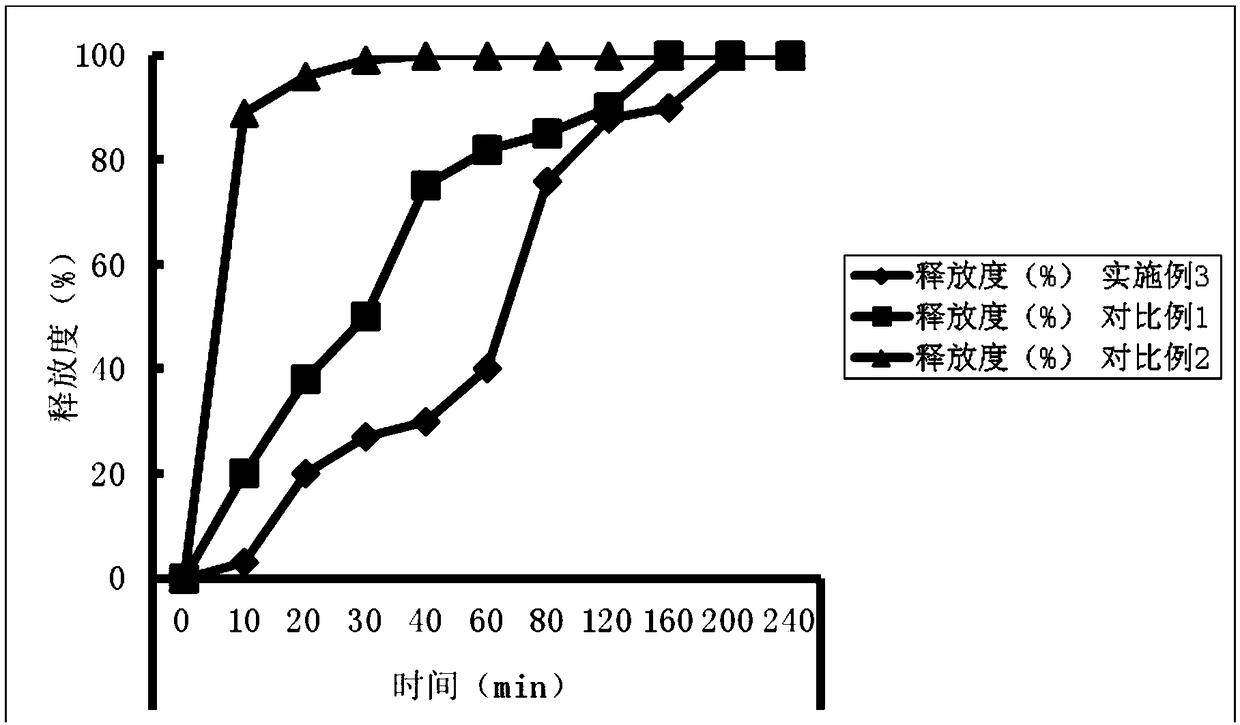
Crisaborole ointment and preparation method thereof
PendingCN110464702AImprove uniformityImprove stabilityAerosol deliveryBoron compound active ingredientsMedicineCrisaborole
The invention belongs to the technical field of pharmaceutical preparations, and specifically relates to an external Crisaborole preparation; and especially, the invention relates to Crisaborole ointment and a preparation technology thereof. The Crisaborole ointment disclosed by the invention contains no water in the components, and comprises the following ingredients in percentage by weight:
Owner:SHANGHAI JIANHE PHARM & TECH CO LTD
Preparation method of crisaborole
The invention relates to the technical field of crisaborole, in particular to a preparation method of crisaborole. The method comprises the following steps: carrying out nitration reaction on cyanophenylboronic acid and concentrated nitric acid to obtain 2-cyan-4-nitrobenzeneboronic acid; then carrying out reduction reaction to obtain 2-formoxyl-4-nitrobenzeneboronic acid; then carrying out reduction reaction to obtain 2-hydroxymethyl-4-nitrophenylboronic acid; carrying out condensation reaction to obtain 2-hydroxymethyl-5-nitrobenzeneboronicacidhemiester; carrying out reduction reaction and diazotization hydrolysis reaction to obtain 2-hydroxymethyl-5-hydroxy benzeneboronicacidhemiester; and finally, carrying out etherification reaction on the 2-hydroxymethyl-5-hydroxy benzeneboronicacidhemiester and fluorobenzonitrile to obtain the crisaborole. The preparation method of the crisaborole has the advantages that the purity of the prepared crisaborole is good, and the yield is high.
Owner:ANHUI QINGYUN PHARMA & CHEM
Compound pharmaceutical composition for treating inflammatory diseases of skin
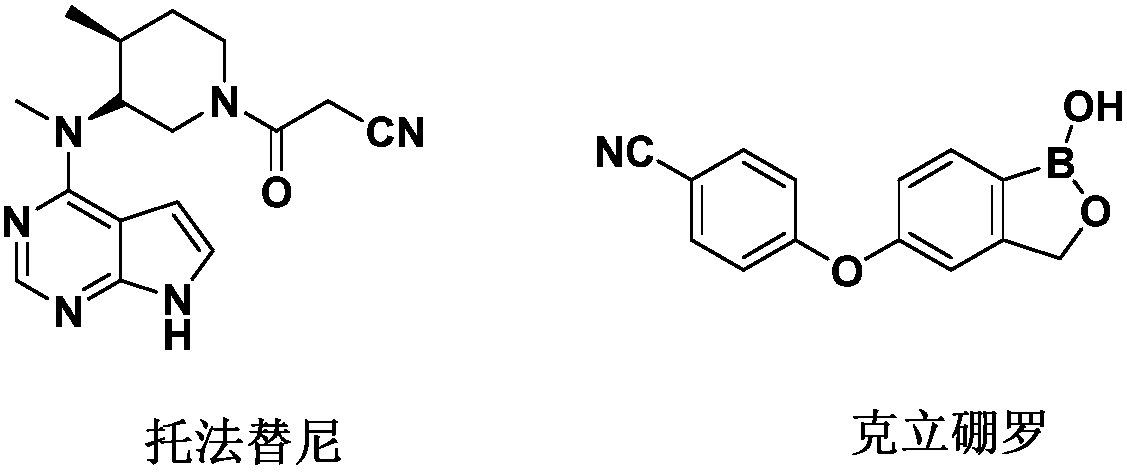
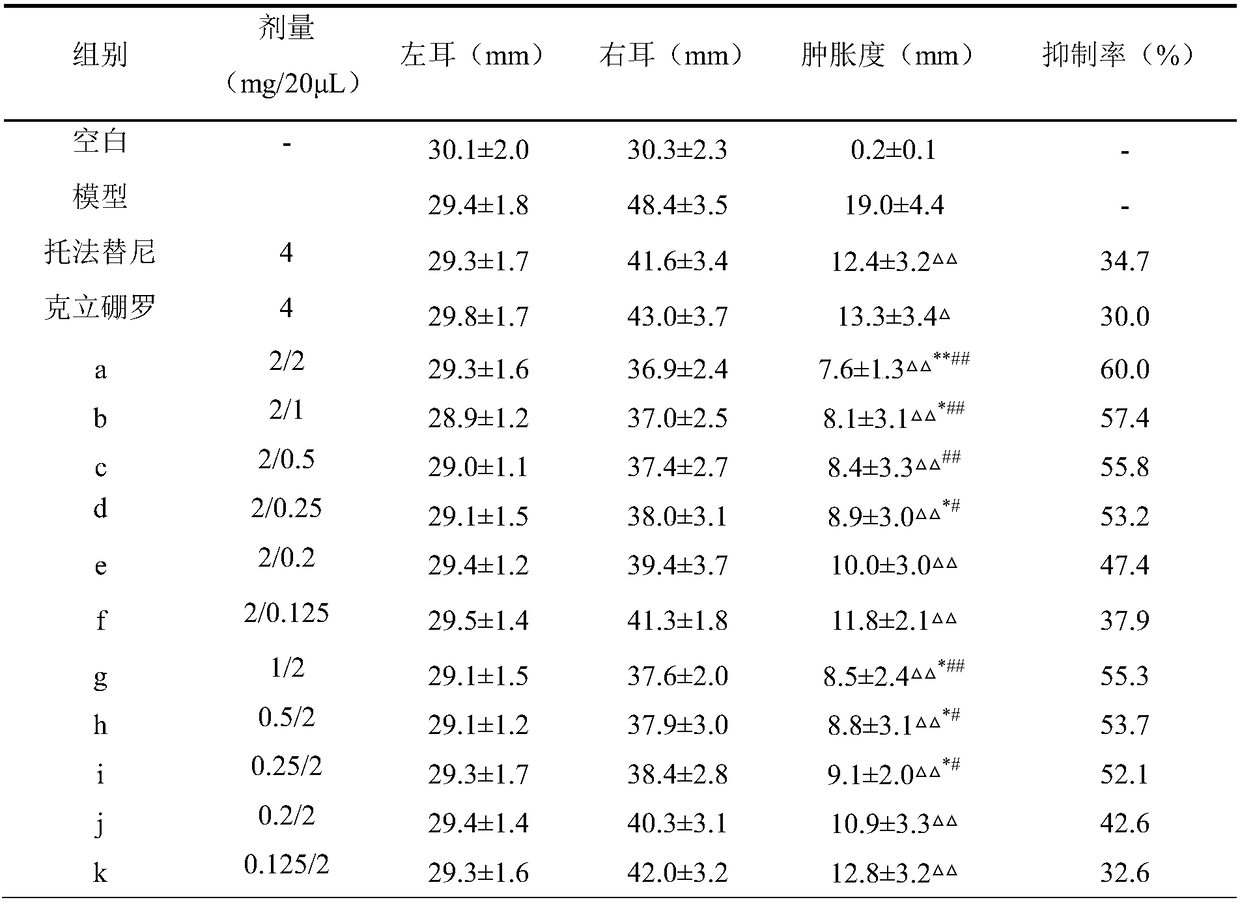
The invention relates to the technical field of medicines, in particular to compound pharmaceutical composition for treating inflammatory diseases of skin. The compound pharmaceutical composition is characterized in that active ingredients of the compound medicine comprise tofacitinib and crisaborole. The pharmaceutical composition has higher treatment effects and lower use dosage, has remarkablecollaborative treatment effects and can be used for treating inflammatory diseases of skin.
Owner:HEFEI IND PHARMA INST +1
Preparation method of Crisaborole intermediate
InactiveCN108997399ALow priceMild reaction conditionsGroup 3/13 element organic compoundsTert-butyldimethylsilyl chlorideTrimethyl borate
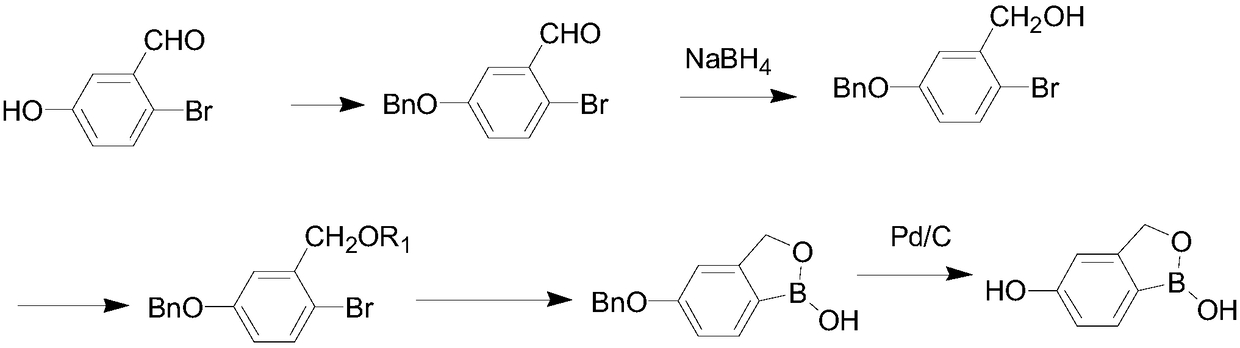
The invention discloses a preparation method of a Crisaborole intermediate. The Crisaborole intermediate has a structure shown as the formula VI. The preparation method comprises the following steps:performing a contact reaction on a compound shown as the formula I and benzyl halide, so as to form a compound shown as the formula II; performing a contact reaction on the compound shown as the formula II and alkali metal borohydride, so as to obtain a compound shown as the formula III; performing a contact reaction on the compound shown as the formula III and a compound a, or performing a contact reaction on the compound shown as the formula III and dihydropyran, so as to obtain a compound shown as the formula IV, wherein the compound a is trimethylchlorosilane, tert-butyldimethylsilyl chloride and chloromethyl methyl ether; performing a contact reaction on the compound shown as the formula IV and an isopropylmagnesium chloride solution; adding an obtained solution into a compound b, performing a contact reaction on the mixture and fourth organic solvent mixed liquor and adding hydrochloric acid into the mixture for contact reaction, so as to obtain a compound shown as the formula V,wherein the compound b is 2-alkoxy-4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborolane, triisopropyl borate or trimethyl borate; performing a hydrogenation reaction on the compound shown as the formula V toobtain a compound shown as the formula VI.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Crisaborole temperature-sensitive gel as well as preparation method and application thereof
InactiveCN108451898AGood sustained release effectPrevent extravasationAerosol deliveryBoron compound active ingredientsGlycerolButylated hydroxytoluene
The invention provides crisaborole temperature-sensitive gel as well as a preparation method and application thereof. The crisaborole temperature-sensitive gel is prepared from the following raw material components in percentage by mass: 1 to 2 percent of crisaborole, 17 to 23 percent of poloxamer, 0.1 to 1 percent of modified cellulose, 5 to 15 percent of propylene glycol and / or glycerol, 5 to 10percent of ethanol, 0.01 to 0.02 percent of butylated hydroxytoluene and the balance of water. The crisaborole temperature-sensitive gel disclosed by the invention has the advantages that injection implantation and long-term drug release can be achieved; the drug can play a good slow-release effect in a human body by adopting a percutaneous drug delivery mode; in addition, drug extravasation canbe prevented and long-term drug release is achieved.
Owner:苏州尚宜佳生物科技有限公司
Process for the preparation of crisaborole and its intermediates
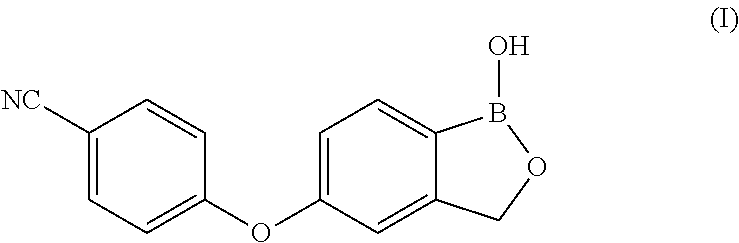
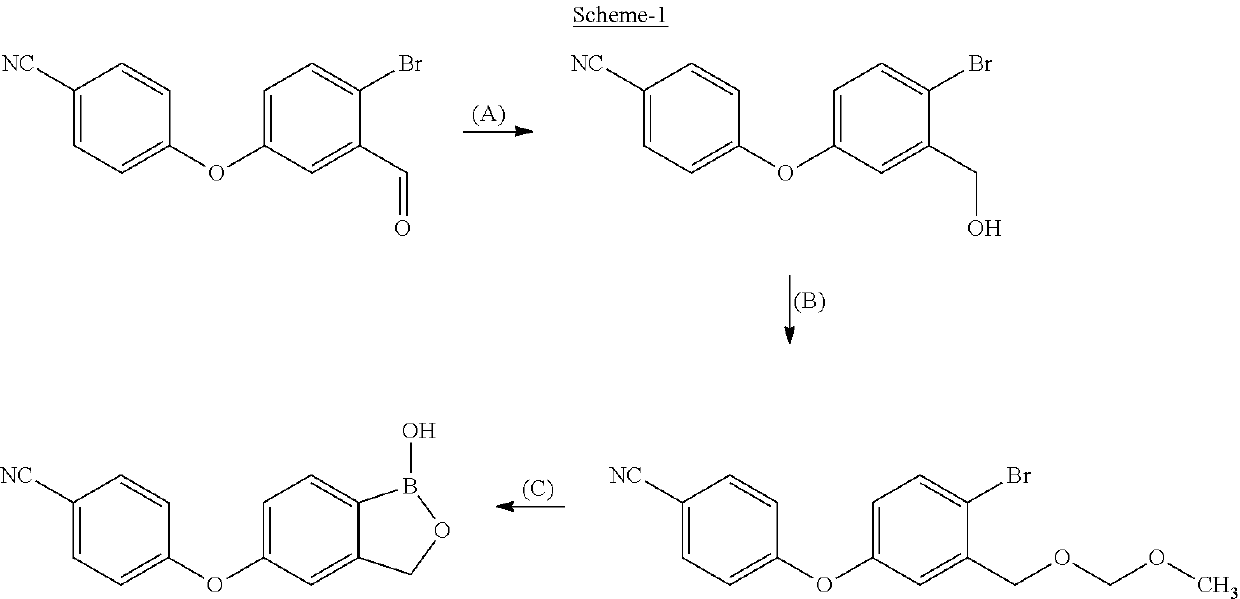
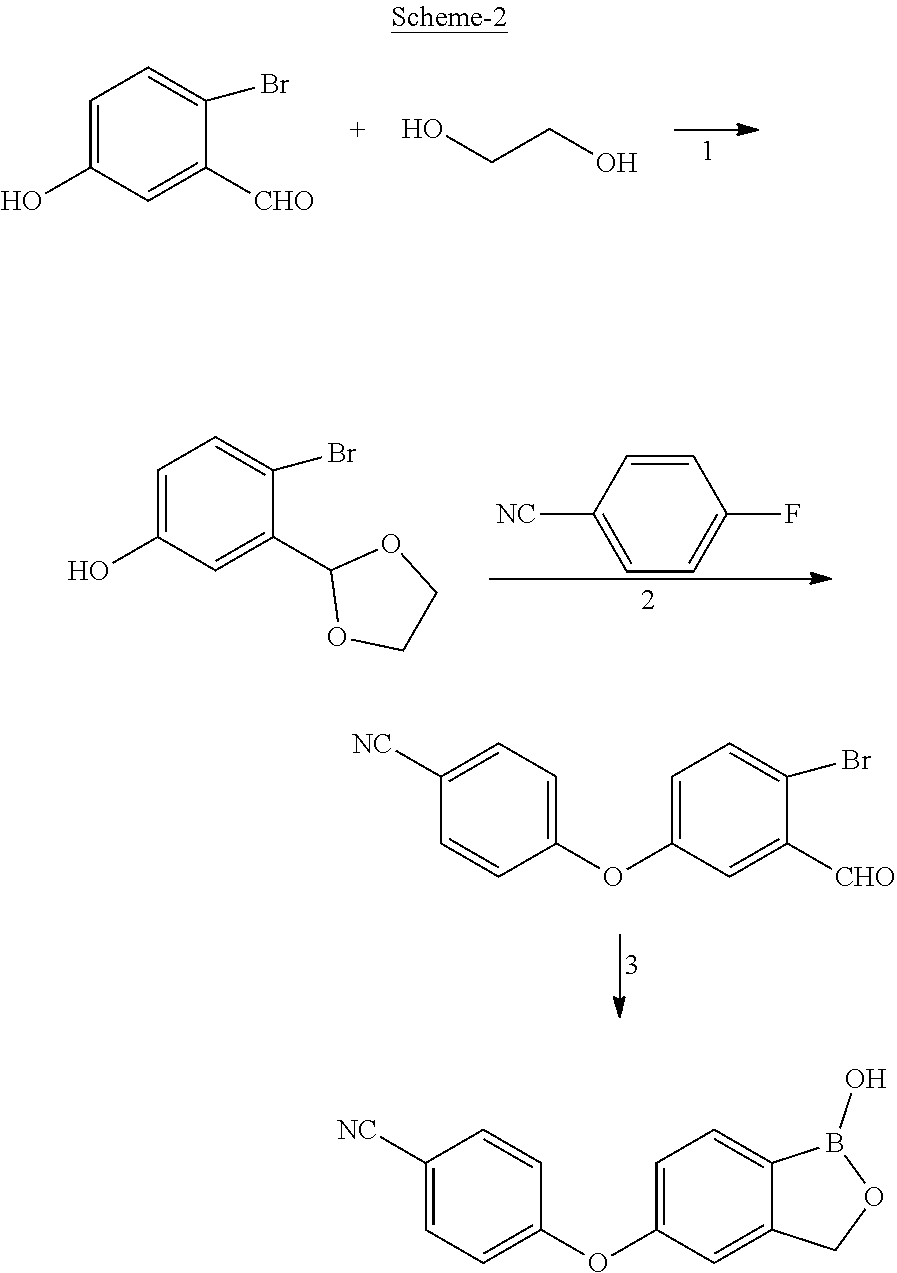
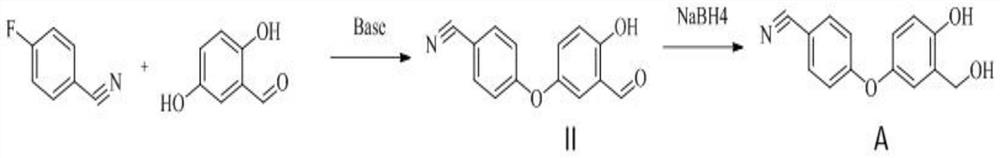
ActiveUS11014944B2Speed up the processMitigate such drawbackOrganic compounds purification/separation/stabilisationCarboxylic acid nitrile preparationCombinatorial chemistryPharmaceutical medicine
The present invention provides a novel and improved process for the preparation of Crisaborole of Formula (I) and its pharmaceutically acceptable salts. The present invention also provides novel intermediates and process for the preparation of intermediates used in the preparation of Crisaborole. The present invention also provides an improved process for the preparation of Crisaborole and pharmaceutically acceptable salts thereof, that is commercially and industrially scalable.
Owner:HALCYON LABS PTE LTD
Preparation method of crisaborole impurity
PendingCN114716349AHigh purityEasy to prepareOrganic compound preparationChemical recyclingBenzoic acidFluid phase
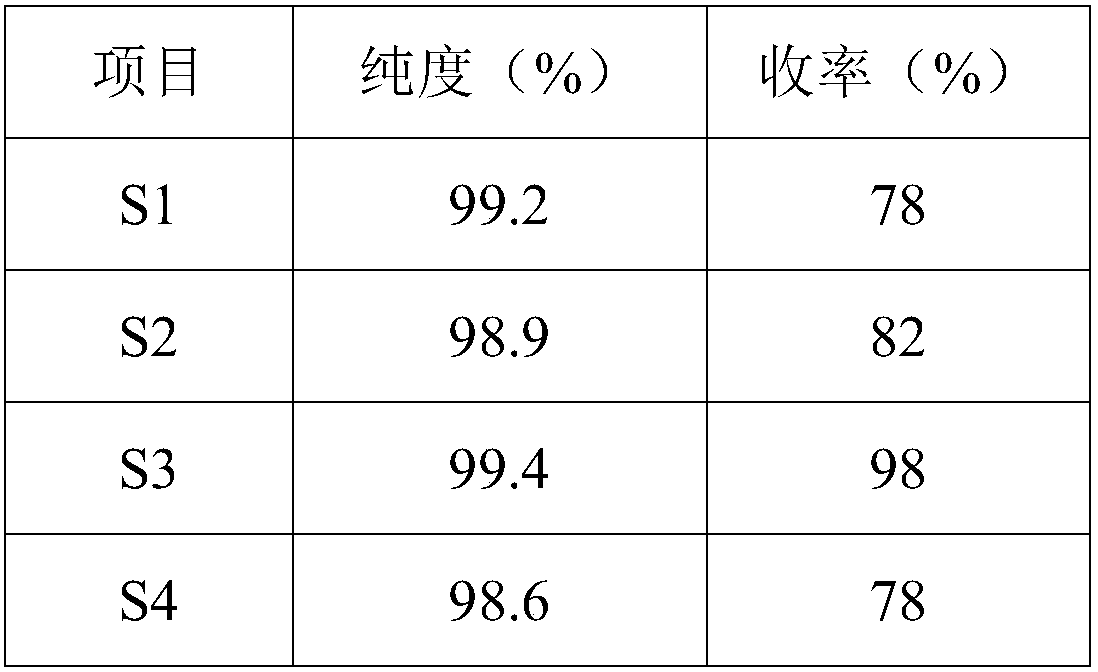
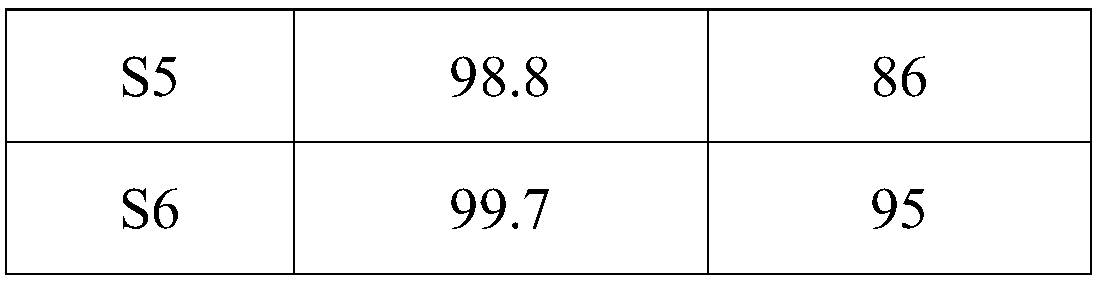
The invention relates to a preparation method of a crisaborole impurity A. The preparation method comprises the following steps: S1, taking 2, 5-dihydroxybenzoic acid and p-halobenzonitrile as raw materials to react, and after the reaction is completed, separating reaction liquid to generate a crisaborole impurity II crude product; s2, purifying the crisaborole impurity I crude product by adopting liquid phase preparation to obtain a refined product; and S3, reducing the crude product of the crisaborole impurity II by using sodium borohydride, and after the reaction is completed, separating and purifying the reaction liquid to obtain the crisaborole impurity A. The preparation method has the advantages that 2, 5-dihydroxybenzoic acid and p-halobenzonitrile are used as raw materials, the raw materials react to generate a crisaborole impurity II, the crisaborole impurity II is purified and then reduced by hydroboration, and the crisaborole A is obtained, so that the preparation method is simple, and the obtained impurity A is high in purity and can be used as a standard substance for the quantitative detection process of crisaborole A; the used reagent raw materials are cheap and easy to obtain, and the crisaborole impurity II with high purity can be prepared in the synthesis route.
Owner:武汉绿合医药科技有限公司
Method for preparing Crisaborole
ActiveUS11214581B2Readily availableNo dangerGroup 3/13 element organic compoundsMethyl benzenePhenol
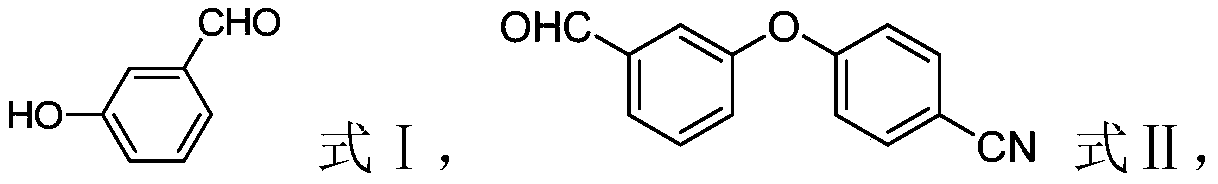
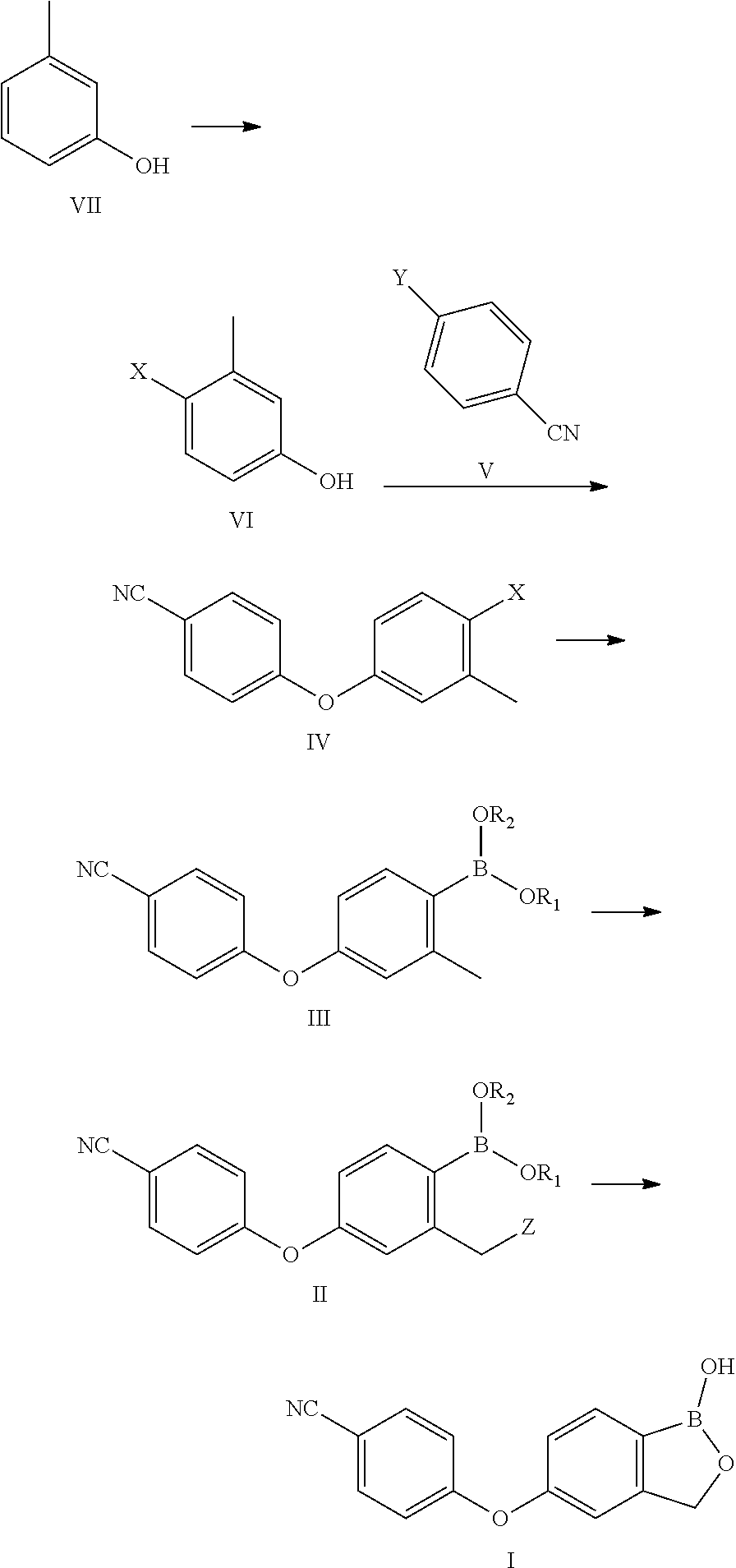
The invention relates to a method for preparing Crisaborole of Formula I, comprising using m-methylphenol as the starting material to obtain a target product through a five-step reaction. The starting materials and the raw materials used in each step of the method according to the present invention are cheap and easy to obtain, and the process is simple. The reaction of introducing boron atoms into the benzene ring to form an oxygen boron heterocycle is novel, with high yield and mild conditions, and is suitable for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Preparation method of crisaborole
ActiveCN112724167AEasy to getLow costGroup 3/13 element organic compoundsBulk chemical productionHydrolysisHydroxy group
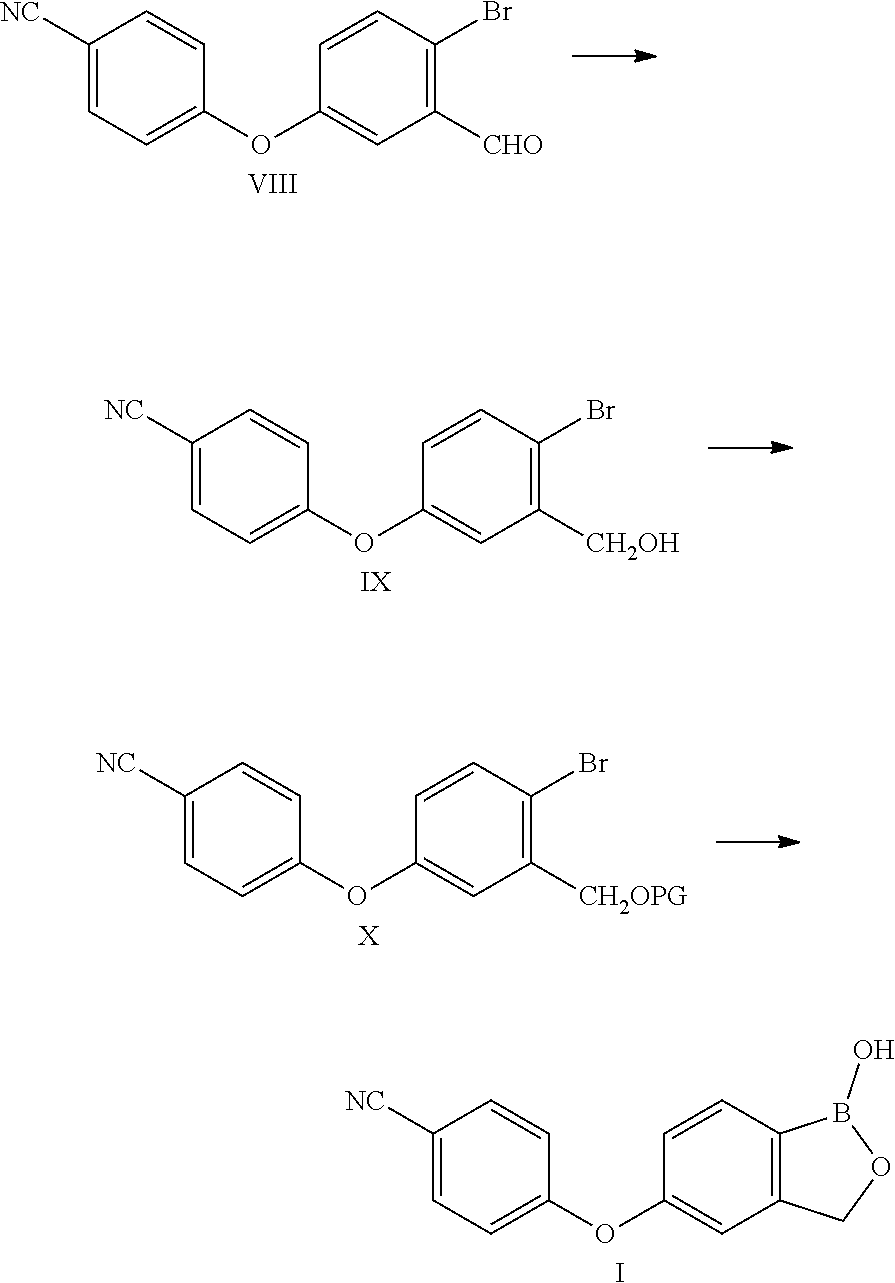
The invention provides a preparation method of crisaborole. More specifically, 5-bromophthalide is used as an initial raw material, and is subjected to etherification, hydrolysis, hydroxyl protection, condensation reaction, photoreaction boronation and cyclization to obtain the crisaborole. The preparation method has the advantages of readily available raw materials, mild reaction conditions, avoidance of metal catalysis and harsh conditions due to adoption of photoreaction for boronizing, low cost, convenience in operation and suitability for industrial production.
Owner:HUBEI HENGAN PHARMA
Preparation method of crisaborole
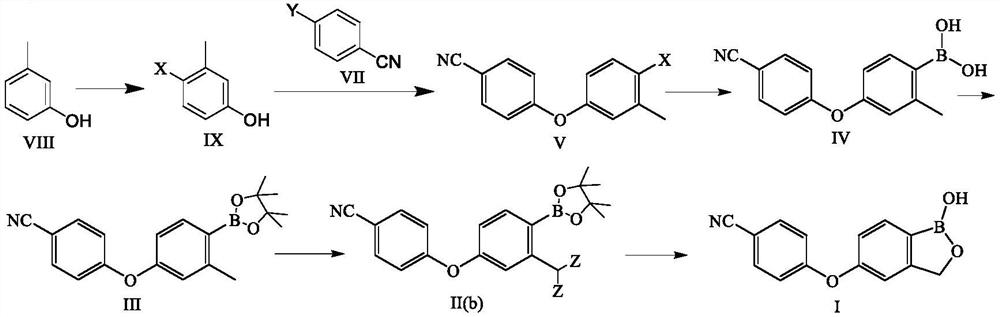
PendingCN113336781AStarting materials are cheap and readily availableMild responseGroup 3/13 element organic compoundsHalogenMethyl benzene
The invention discloses a preparation method of crisaborole. The preparation method comprises the following steps: by taking m-methylphenol as a raw material, firstly coupling the m-methylphenol with 4-halogenated-cyanophenyl, and then under the action of a halogenating reagent, increasing the yield of an intermediate product 4-(4-halogen-3-methylphenoxy) cyanophenyl; and carrying out halogen-metal exchange, halogenation, ring closing and other reactions to prepare the crisaborole. Compared with the prior art, the method has advantages that the raw materials are cheap and easy to obtain, and expensive reagents and raw materials in the prior art are not used; the reaction is mild, and ultralow-temperature reaction in the prior art can be avoided; the method is short in reaction time, free of column chromatography purification, simple in process, convenient to operate and suitable for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Method for preparing crisaborole
InactiveCN109517003ARaw materials are easy to getSimple stepsCarboxylic acid nitrile preparationOrganic compound preparationSolventStructural formula
The invention discloses a method for preparing crisaborole shown as a formula I. The preparation method disclosed by the invention comprises the following steps: carrying out the following reaction between a compound IV and bis(pinacolato)diboron in a solvent in the presence of alkali and a catalyst, thereby obtaining the compound V. The preparation method disclosed by the invention has the characteristics of being readily available in raw materials, simple in steps, mild in reaction conditions, controllable in quality, environmental-friendly, low in cost and the like. The industrial production of the bulk drug is facilitated, and development of the economic technology is promoted. The structural formula is as shown in the specification.
Owner:成都安满生物医药科技有限公司
Crisaborole intermediate preparation method
PendingCN110467544ALow priceShort preparation stepsCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventOrtho position
The invention discloses a crisaborole intermediate preparation method, wherein the crisaborole intermediate has a structure represented by a formula IV. The preparation method comprises: in the presence of a first organic solvent and a first alkali, carrying out a contact reaction on a compound represented by a formula I and p-fluorobenzonitrile to obtain a compound represented by a formula II; inthe presence of a second solvent, carrying out a contact reaction on the compound represented by the formula II and an alkali metal borohydride to obtain a compound represented by a formula III; andin the presence of a third organic solvent and a catalyst, carrying out a contact reaction on the compound represented by the formula III and a bromination reagent to obtain a compound represented bya formula IV. According to the present invention, with the preparation method, the reaction raw material p-fluorobenzonitrile is used as the ortho-position steric hindrance group, such that the use ofprotective groups is eliminated, the selectivity of the bromination reaction is improved, the process cost is low, the raw material price is cheap, and the method can be well used for industrial production.
Owner:WUHAN POLYTECHNIC UNIVERSITY
A kind of preparation method of crisborole
ActiveCN108047261BMild reaction conditionsEasy to operateGroup 3/13 element organic compoundsBulk chemical productionPhenylboronic acidPtru catalyst
The invention discloses a method for preparing crisaborole and an intermediate for preparing crisaborole. The method comprises the following steps: (1) enabling components such as 2-halogen-5-(4-cyanphenoxy) benzyl acetate and bis(pinacolato)diboron to react in the presence of an organic solvent and a palladium catalyst under an alkali condition so as to obtain components such as 2-acetoxyl methyl-4-(4-cyan phenoxy) phenylboronic acid pinacol ester; (2) enabling components such as the 2-acetoxyl methyl-4-(4-cyan phenoxy) phenylboronic acid pinacol ester to react in a solvent under an acid oralkali condition, thereby obtaining crisaborole. The method is gentle in reaction condition, easy to operate, high in reaction yield, simple in aftertreatment, relatively low in cost and applicable toindustrial production.
Owner:SUZHOU VIGONVITA LIFE SCIENCES CO LTD +1
Crisaborole sustained-release film with good paste effect and preparation method thereof
InactiveCN108926547AImprove bindingMake up for the shortcomings of poor bonding effectAntimycoticsBoron compound active ingredientsPlasticizerAdhesive
The invention discloses a Crisaborole sustained-release film with good paste effect and a preparation method thereof. The film is prepared by the following raw materials: active ingredients, a plasticizer, a filler, an absorption enhancer, a suspending agent, and an adhesive. The film solves the problem that the frequency of drug use is too high and a longer skin retention time and a reduced number of administrations can be provided, a film preparation is prepared by using a variety of film-forming auxiliary materials, which complement each other and indispensable, the plasticizer and the filler enhance film formation, the absorption enhancer enhances drug penetration capability, and the suspending agent ensures the dispersibility of the raw materials in a liquid state, and the adhesive enhances the adhesion of the film to the skin.
Owner:苏州尚宜佳生物科技有限公司
Preparation method of 2-formyl-4-(4-cyanophenoxy) phenylboronic acid pinacol ester
ActiveCN113336780AHigh yieldHigh purityGroup 3/13 element organic compoundsPtru catalystBoronic acid
The present invention relates to a novel process for the preparation of 4-[3-formyl-4-(4, 4, 5, 5-tetramethyl-1, 3, 2-dioxaborol-2-yl) phenoxy] benzonitrile; and the compound is used as an intermediate for the synthesis of crisaborole. The method adopted by the invention is a non-palladium-catalyzed decarboxylation boronation method which takes carboxylic ester as a substrate and isonicotinate as a catalyst; and compared with a palladium-catalyzed coupling boronation method which takes halide as a substrate in the prior art, the method has the advantages of safety in operation, low cost, environmental friendliness and the like.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES +1
Crisaborole prodrug and its preparation method and use
ActiveCN112824418BImprove solubilityImprove bioavailabilityNervous disorderAntipyreticEfficacyBlood plasma
The present invention obtains a series of Crisaborole prodrugs represented by the general formula I by modifying the active site of Crisaborole: the compound designed by the present invention can be rapidly converted into the original drug Crisaborole in plasma, and compared with the original drug, it has better It has better solubility, higher bioavailability, enhanced efficacy, and is a new type of compound with PDE‑4 inhibitory activity.
Owner:苏州东南药业股份有限公司
A crisaborole sustained-release film having good absorption effects and a preparing method thereof
InactiveCN108938602AImprove permeabilityImprove fitAntimycoticsBoron compound active ingredientsPlasticizerActive component
A crisaborole sustained-release film having good absorption effects and a preparing method thereof are disclosed. The film is prepared from an active component, a plasticizer, a filler, an absorptionpromoter, a suspending aid and an adhesive. The film can overcome a problem that medicine use frequency is over-high and can provide longer skin residence time and a decreased number of times of administration. A plurality of film forming auxiliary materials, which supplement each other and which are integral, are adopted to prepare the film, the plasticizer and the filler enhance film forming performance, the absorption promoter enhances medicine infiltration capacity, the suspending aid ensures dispersibility of raw materials in a liquid state, and the adhesive enhances the degree of adhesion between the film and skin.
Owner:苏州尚宜佳生物科技有限公司
Medicinal gel, preparation method thereof and application of medicinal gel
InactiveCN112370420AGood sustained release effectPrevent extravasationAerosol deliveryOintment deliveryCelluloseHuman body
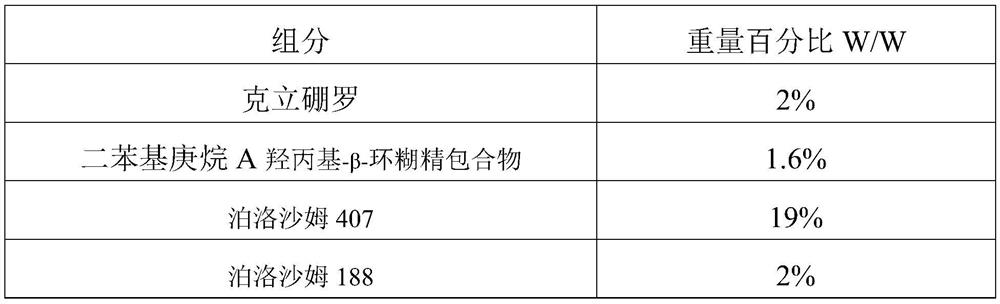
The invention discloses a medicine gel, a preparation method thereof and application of the medicine gel. The medicine gel is prepared from the following components: 1-2 wt% of crisaborole, 1.2-2.4 wt% of diphenylheptane A hydroxypropyl-beta-cyclodextrin inclusion compound, 18-20 wt% of poloxamer 407, 1-3 wt% of poloxamer 188, 0.1-0.2 wt% of hydroxypropyl cellulose, 8-10 wt% of propylene glycol, 5-10 wt% of glycerol, 0.1-0.2 wt% of ethylparaben and 51.6-64.9 wt% of water. The medicinal gel disclosed by the invention can realize long-term drug release; By employing a percutaneous drug deliveryway, the drug can play a very good slow-release role in a human body, and the drug can be prevented from extravasation, and is released for a long time.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Preparation method of crisaborole and intermediate product thereof
PendingCN113979891AImprove securityMild reaction conditionsCarboxylic acid nitrile preparationOrganic compound preparationOrganic solventPtru catalyst
The invention discloses a preparation method of a crisaborole intermediate product. The crisaborole intermediate product has a structure represented by a general formula A1. The preparation method comprises the following steps: S1, adding a compound represented by a chemical formula II into an organic solvent, adding an acidic material, and carrying out a reaction to obtain a compound represented by a chemical formula III. The invention also discloses a preparation method of the crisaborole, which comprises the preparation method of the intermediate product of the crisaborole, and further comprises the following steps: adding the compound represented by a chemical formula VI into an organic solvent, carrying out contact reaction on the compound and bis (pinacolato) diboron under an alkaline condition and a palladium catalyst, and cyclizing under an acidic condition to obtain the crisaborole. According to the present invention, the yield and the safety performance of the whole process can be improved, the process production process is optimized, the excellent mass production method is provided for the crisaborole, and the crisaborole can be well applied to the industrial production.
Owner:SHANGHAI GAOZHUN PHARMA CO LTD
A crisaborole sustained-release film having good sustained release effects and a preparing method thereof
InactiveCN108938603AIncrease concentrationSustained release therapy works wellAntimycoticsBoron compound active ingredientsActive componentAdhesive
A crisaborole sustained-release film having good sustained release effects and a preparing method thereof are disclosed. The film is prepared from an active component, a plasticizer, a filler, an absorption promoter, a suspending aid and an adhesive. The film can overcome a problem that medicine use frequency is over-high and can provide longer skin residence time and a decreased number of times of administration. A plurality of film forming auxiliary materials, which supplement each other and which are integral, are adopted to prepare the film, the plasticizer and the filler enhance film forming performance, the absorption promoter enhances medicine infiltration capacity, the suspending aid ensures dispersibility of raw materials in a liquid state, and the adhesive enhances the degree ofadhesion between the film and skin.
Owner:苏州尚宜佳生物科技有限公司
Method for synthesizing crisaborole intermediate by using microchannel reactor
InactiveCN112759605AContinuous online mixingLow impurity contentChemical/physical/physico-chemical microreactorsGroup 3/13 element organic compoundsHydrogenation reactionPhysical chemistry
The invention discloses a method for synthesizing a crisaborole intermediate by using a microchannel reactor. The method comprises the following steps: dissolving an intermediate a in an organic solution b, uniformly mixing to obtain a material 1, and conveying the material 1 to a pre-cooling module in the microchannel reactor through a plunger pump for mixing and pre-cooling. According to the present invention, the crisaborole intermediate is synthesized by using the microchannel reactor, the continuous online mixing, pre-cooling and reaction of the reaction material liquid can be achieved due to the unique microstructure design of the microchannel reactor, and the mixing reaction can be completed in the short time even if the two-phase or the three-phase is not dissolved, compared with the traditional stirring hydrogenation reaction kettle, the mixing efficiency is improved by more than 100 times, the whole reaction time can be shortened from several hours to about 30 seconds, the impurity content of the product can be greatly reduced due to overhigh local concentration in the process, the purity and the yield of the product are improved, and the safety is also greatly improved.
Owner:HEFEI LIFEON PHARMA
Pharmaceutical composition, pharmaceutical preparation and application of pharmaceutical composition and pharmaceutical preparation
InactiveCN112494501AFacilitated releaseSignificant anti-inflammatory synergyAntipyreticAerosol deliveryPharmaceutical drugCombinatorial chemistry
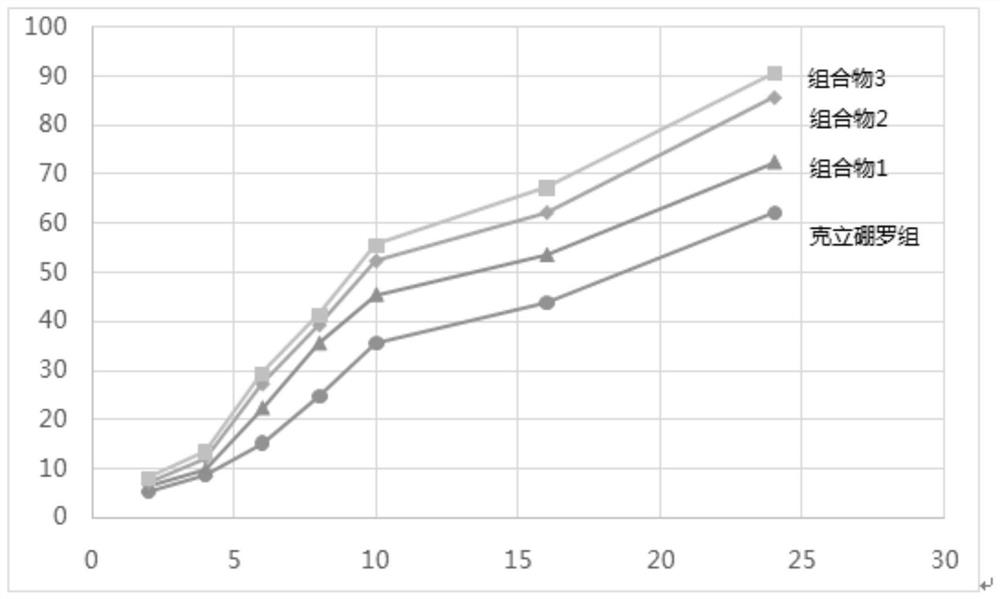
The invention discloses a pharmaceutical composition, a pharmaceutical preparation and application of the pharmaceutical composition and the pharmaceutical preparation. The pharmaceutical compositioncomprises Crisaborole and diphenylheptane A. Compared with the Crisaborole, the pharmaceutical composition disclosed by the invention has better transdermal performance and anti-inflammatory effect.
Owner:WUHAN POLYTECHNIC UNIVERSITY
Crisaborole dampness eliminating diuretic sustained-release membrane and preparation method thereof
InactiveCN108785372AEvenly dispersedGood sustained release effectAntimycoticsAerosol deliveryMatrineMedicine
The invention discloses a crisaborole dampness eliminating diuretic sustained-release membrane and a preparation method thereof. The membrane is prepared by the following raw materials: 9-18mg of crisaborole, 0.2-1.2mg of matrine extract, 0.2-1.2mg of garden balsam stem extract, 1-2.5mg of modified starch, 0.6-1.0mg of carbopol and 4-10mg of glycerol. The problem that the frequency of drug use istoo high is solved, longer skin retention time can be achieved, and the dosing times can be reduced. The resistant starch has good gelation property but loose structure. The addition of the carbopol and the glycerin can improve the structural strength, so that the materials are prone to form the membrane, and the yield is improved.
Owner:苏州尚宜佳生物科技有限公司
Application of phosphodiesterase inhibitor or pharmaceutical composition thereof in preparation of medicines for treating novel coronavirus pneumonia
PendingCN114053280APrevent proliferationStrong anti-coronavirus effectBoron compound active ingredientsAntiviralsZardaverinePhosphodiesterase inhibitor
The invention provides the application of a phosphodiesterase inhibitor or a pharmaceutical composition of the phosphodiesterase inhibitor in preparation of medicines for treating novel coronavirus pneumonia, screening of systematic biology and cellular level experiments verify that zardaverine, Pimobendan and crisaborole can inhibit proliferation of novel coronavirus to different degrees on the cellular level; wherein zardaverine has the strongest anti-new coronavirus effect, Pimobendan has the second anti-new coronavirus effect, and zardaverine, Pimobendan and crisaborole can be used for preparing medicines for treating novel coronavirus pneumonia.
Owner:SOUTH UNIVERSITY OF SCIENCE AND TECHNOLOGY OF CHINA +1
Preparation method of crisaborole intermediate
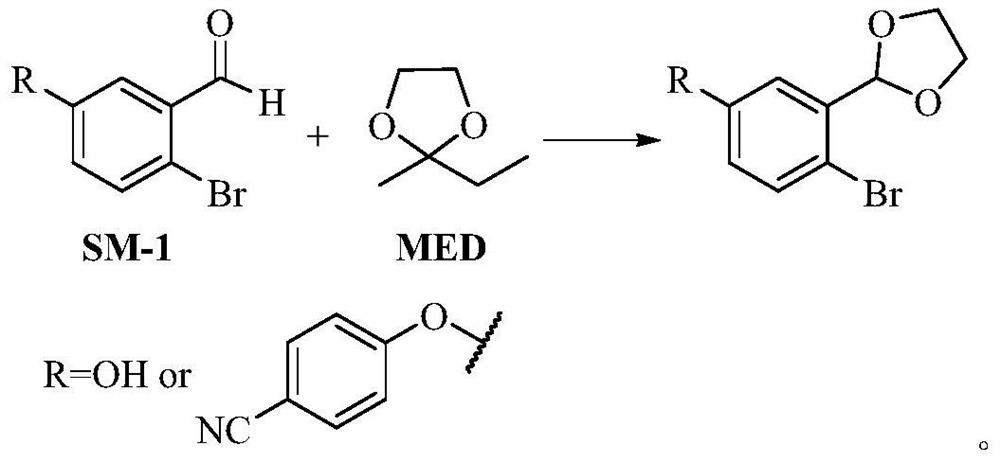
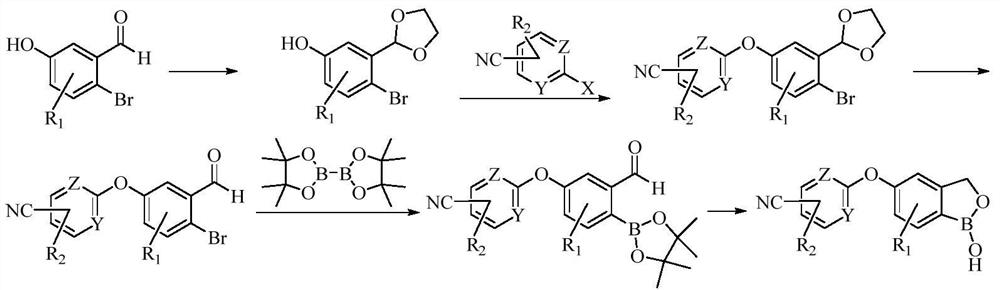
PendingCN113801089AReduce usageLow reaction temperatureOrganic chemistryBiochemical engineeringDrugs synthesis
The invention belongs to the technical field of medicine synthesis, and particularly relates to a preparation method of a crisaborole intermediate. MED is used for replacing ethylene glycol in the prior art for acetal protection, so that the reaction temperature and the use of a water segregator can be obviously reduced, the reaction operation is simplified, and the energy consumption is reduced. Compared with the prior art, the preparation process provided by the invention has the advantages that the product yield and purity are higher, and the preparation process is more suitable for industrial production.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of skin-touch crisaborole slow-release film
InactiveCN108853062AImprove permeabilityImprove fitAntimycoticsBoron compound active ingredientsDispersityAdhesive
The invention discloses a preparation method of a skin-touch crisaborole slow-release film. The skin-touch crisaborole slow-release film is prepared from the following raw materials: an active component, a plasticizer, a filling agent, an absorption promoter, a suspending agent and an adhesive. According to the preparation method disclosed by the invention, the problem that the utilization frequency of a drug is too high is solved, longer skin retention time is provided and the number of times of drug administration is reduced; a film agent is prepared by matching various film-forming auxiliary materials and the materials are complementary to each other and are necessary; the film forming property is enhanced through the plasticizer and the filing agent and the penetration capability of the drug is enhanced through the absorption promoter; the dispersity of the raw materials under a liquid state is ensured through the suspending agent and the fitting degree between the film agent and skin is enhanced through the adhesive.
Owner:苏州尚宜佳生物科技有限公司
Method for preparing crisaborole
ActiveUS20210053995A1Readily availableNo dangerGroup 3/13 element organic compoundsMethyl benzenePhenol
The invention relates to a method for preparing Crisaborole of Formula I, comprising using m-methylphenol as the starting material to obtain a target product through a five-step reaction. The starting materials and the raw materials used in each step of the method according to the present invention are cheap and easy to obtain, and the process is simple. The reaction of introducing boron atoms into the benzene ring to form an oxygen boron heterocycle is novel, with high yield and mild conditions, and is suitable for industrial production.
Owner:JIANGXI SYNERGY PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com