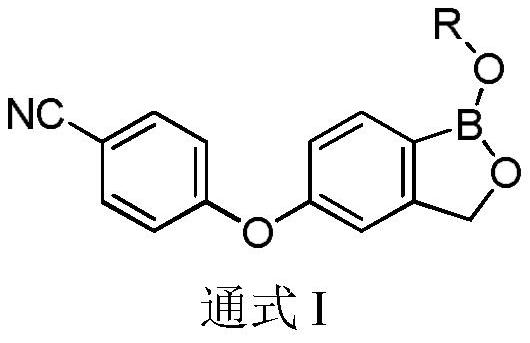
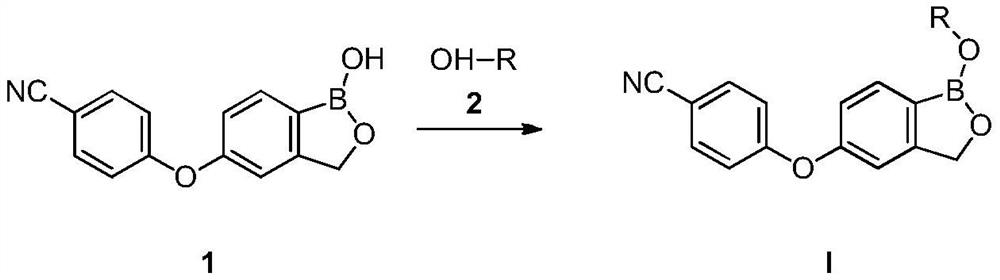
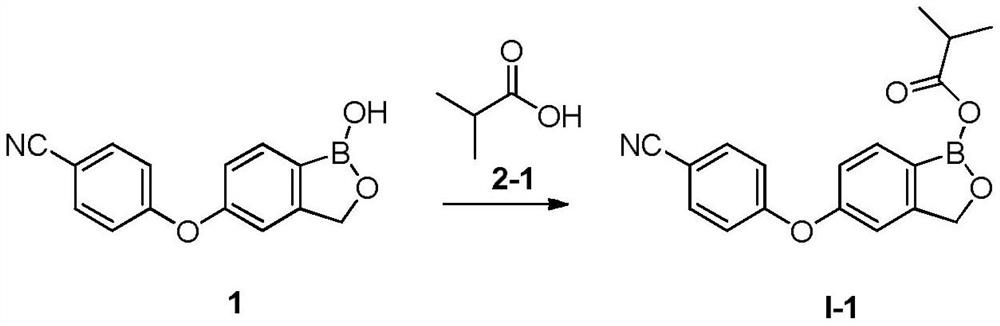
Crisaborole prodrug and its preparation method and use
A pharmacy and CH2 technology, applied in chemical instruments and methods, antipyretics, drug combinations, etc., can solve problems such as side effects and depression, and achieve the effects of enhancing drug efficacy, good solubility, and high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 8
[0058] Example 8 I-1~I-7Crisaborole prodrug compound in vitro stability test
[0059] 1. High performance liquid chromatography determination conditions
[0060] Liquid chromatograph: Waters 2489UV / Visible Detector, Waters 1525Binary HPLC Pump;
[0061] Chromatographic column: Kromasil 100-5-C18, Dim: 4.6x150mm, Part / Serial: M05CLA15 / E121514;
[0062] Mobile phase: acetonitrile (50%-80%) and water gradient elution;
[0063] Flow rate: 1mL / min Column temperature 25°C;
[0064] The detection wavelength is 254nm and the injection volume is 10 μL;
[0065] The retention time of Crisaborole is about 18min without interference from the mobile phase.
[0066] 2. Sample Preparation
[0067] The target compound was dissolved in DMSO solvent, and the concentration was converted to Crisaborole 120 mg / mL according to the concentration. Take 20 μL of this solution and add it to 1.18 mL of fresh rat blank plasma, and incubate at 37 ° C to obtain the sample.
[0068] 3. Sample pretreat...
Embodiment 9
[0079] The above data show that compounds I-1 to I-7 can be rapidly converted into the original drug Crisaborole in plasma. Embodiment 9 Compound I-1~I-7 transdermal drug release experiment
[0080] Tested with the single-compartment static chamber method, the back skin of guinea pigs three months old was taken out successively for the test, and the release pool with the experimental skin piece fixed was installed on the upper side of the single compartment, and the drug was placed on the isolated experimental skin. Constant temperature in a 32°C water bath for a certain period of time, absorb the stirred solution in the subcutaneous compartment of the experiment for measurement, use liquid chromatography and CR2Ax-2 microcomputer processor, liquid chromatography to determine the peak value of samples in the sample chamber and receiving chamber, CR2Ax -2 The microcomputer processor calculates the area of the peak meter, monitors and calculates the drug penetration. The resu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com