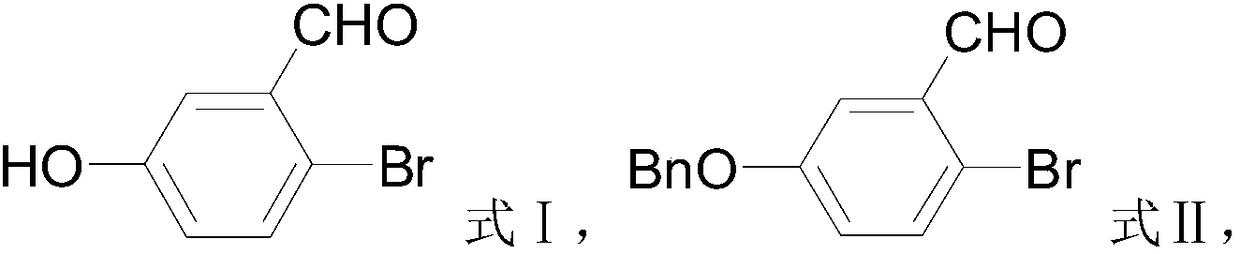Preparation method of Crisaborole intermediate
A technology of criborole and intermediates, which is applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, organic chemistry, etc., can solve the problems of expensive starting materials, and achieve cheap raw materials. , low price, good effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
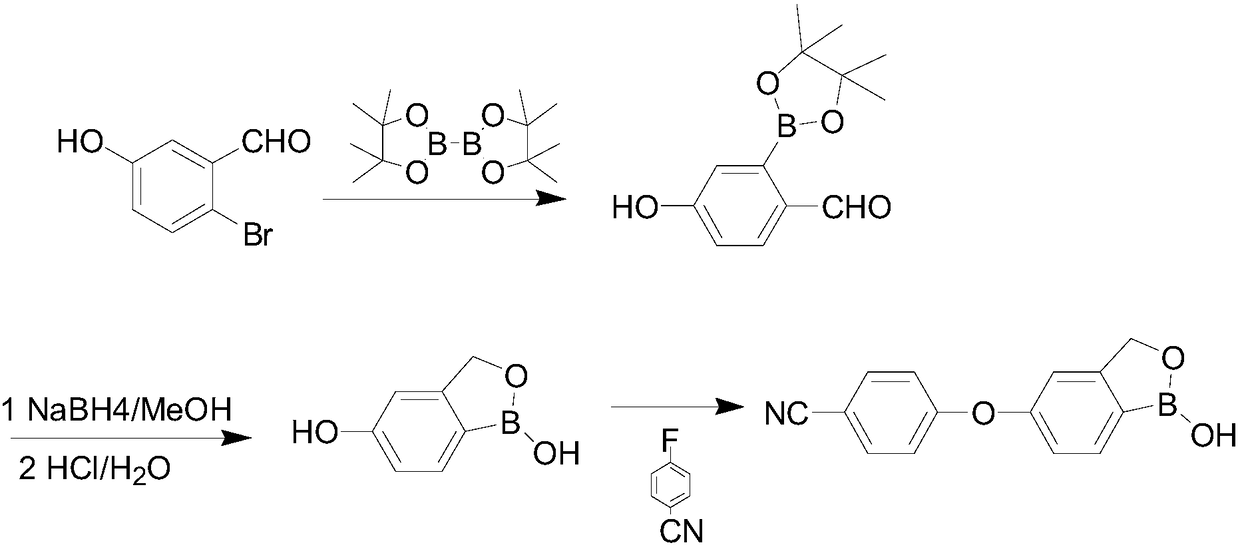
[0027] The invention provides a preparation method of a crisborole intermediate, the crisborole intermediate has a structure shown in formula VI,
[0028] This preparation method comprises the steps:
[0029] (1) In the presence of the first organic solvent and the first base, the compound shown in formula I is contacted with benzyl halide to obtain the compound shown in formula II;
[0030]
[0031] (2) In the presence of a second solvent, the compound represented by the formula II and the alkali metal borohydride are subjected to a contact reaction to obtain the compound represented by the formula III;
[0032]
[0033] (3) In the presence of the third organic solvent and the second base, the compound shown in the formula III and the compound a are subjected to a contact reaction to obtain the compound shown in the formula IV; or in the presence of the third organic solvent and the first catalyst , contacting the compound shown in formula III and dihydropyran to obtai...
Embodiment
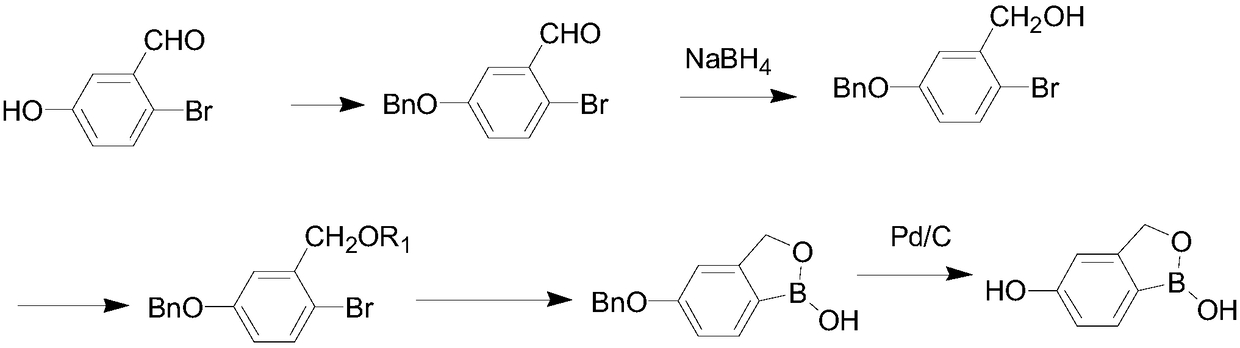
[0051] Such as figure 2 As shown, the present embodiment provides a preparation method of crisborole intermediate, and the specific preparation method is as follows:
[0052] (1) Preparation of 5-benzyloxy-2-bromo-benzaldehyde (compound shown in formula Ⅱ)
[0053] 201g (1mol) 2-bromo-5-hydroxy-benzaldehyde (compound shown in formula I), 1000ml acetone, 276g (1.1mol) of potassium carbonate were added to the reaction kettle, and 152g (1.2mol) of benzyl chloride was added under stirring , refluxed for 10h, recovered the solvent, added 1000ml of water, made a slurry, filtered, and dried under reduced pressure to obtain 279g of solids, with a yield of 96%;
[0054] (2) Preparation of 5-benzyloxy-2-bromo-benzyl alcohol (compound shown in formula III)
[0055] Add 145g (0.5mol) of 5-benzyloxy-2-bromo-benzaldehyde and 2000ml of methanol into the reaction kettle, add 18.9g (1mol) of sodium borohydride in batches, and continue stirring at 25°C for 4h until the raw material After th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com