Preparation method of crisaborole impurity
A creborone and impurity technology, which is applied in the field of preparation of creborone impurities, can solve the problems of difficult separation, impurity of degradation products, and low amount of target products, and achieves the effect of simple preparation method and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
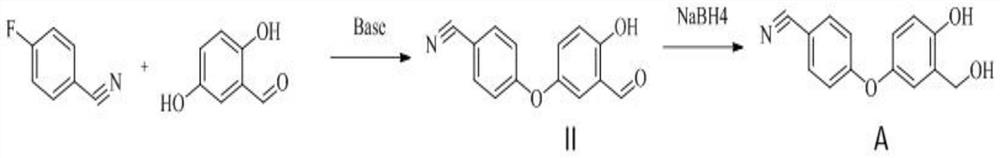
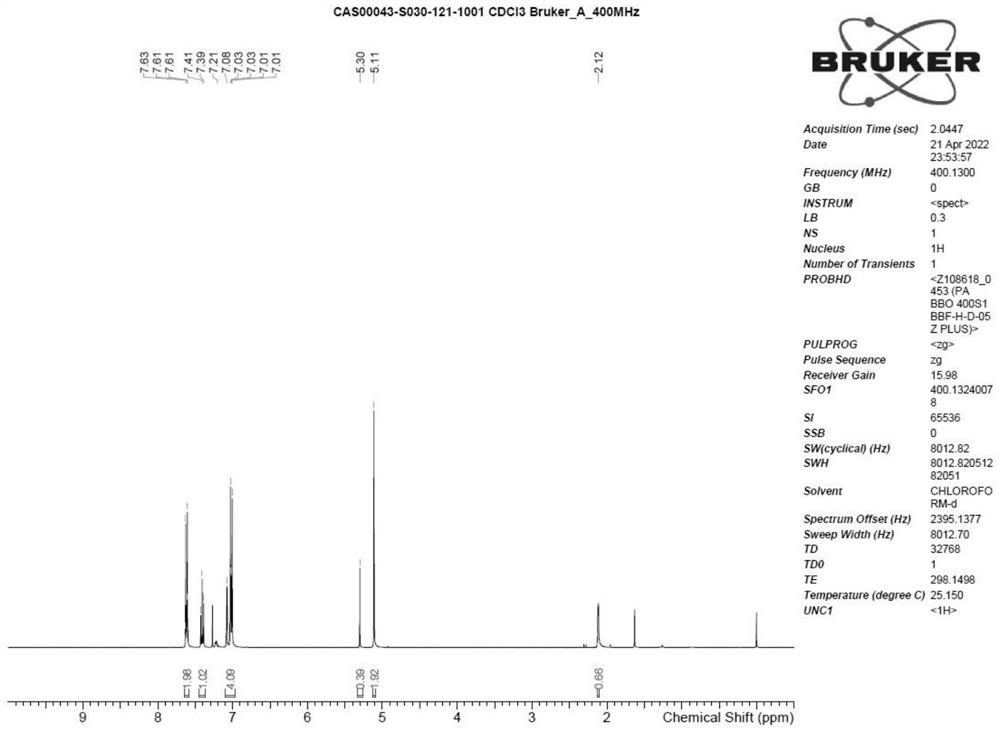
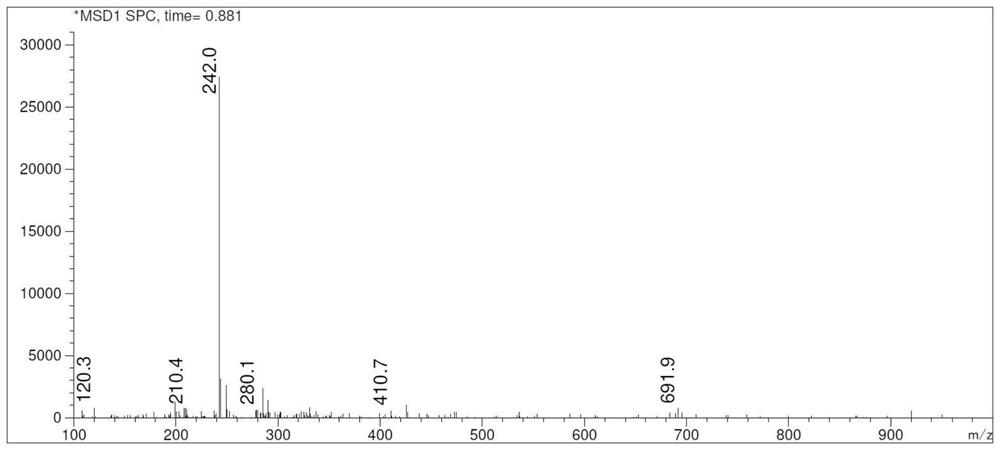
[0034] A kind of preparation method of criborole impurity A, reaction scheme is such as figure 1 shown, it includes the following steps:
[0035] S1. Preparation of Criborone Impurity II:
[0036] Weigh 2,5-dihydroxybenzaldehyde (10.00g, 72mmol), p-fluorobenzonitrile (8.77g, 72mmol), anhydrous K 2 CO 3 (15.01 g, 109 mmol), N,N-dimethylformamide (100 mL) was added, and the mixture was heated to 100° C. and stirred for 5 h. The sampling point board monitors the disappearance of the p-fluorobenzonitrile point, stops heating, and cools down to room temperature. 300 mL of purified water was added, and an oily substance was precipitated. Let stand, pour out the supernatant, add 50 mL of ethyl acetate to the remaining oil, stir and heat to reflux until clear, cool to room temperature and stir for 1-2 h, let stand, pour out the supernatant to obtain viscous solid cleaborol impurity Ⅱ crude product 11.24g.
[0037] S2. Liquid phase preparation and purification:
[0038] Get the ...
Embodiment 2
[0046] A kind of preparation method of Criborone impurity A, it comprises the steps:
[0047] S1. Preparation of Criborone Impurity II:
[0048] Weigh 2,5-dihydroxybenzaldehyde (5.00g, 36mmol), p-fluorobenzonitrile (4.38g, 36mmol), anhydrous K 2 CO 3 (7.50 g, 54 mmol), acetonitrile (50 mL) was added, and the reaction was heated to reflux for 6 h. The sampling point board monitors the disappearance of the p-fluorobenzonitrile point, stops heating, and cools down to room temperature. Suction filtration, and the filtrate was concentrated under reduced pressure to dryness to obtain an oily substance. Add 30 mL of anhydrous ethanol to the oil, stir and heat to 45°C to dissolve, then cool to room temperature after dissolving, slowly add 120 mL of ice water, stir and crystallize for 0.5 to 1 h, filter with suction, and blow dry the filter cake to obtain 6.90 g of a yellow-brown solid.
[0049] S2. Liquid phase preparation and purification:
[0050]Take the yellow-brown solid, ad...
Embodiment 3
[0054] A kind of preparation method of Criborone impurity A, it comprises the steps:
[0055] S1. Preparation of Criborone Impurity II:
[0056] Weigh 2,5-dihydroxybenzaldehyde (3.00g, 22mmol), p-fluorobenzonitrile (3.16g, 26mmol), anhydrous K 2 CO 3 (9.05 g, 65 mmol), added acetonitrile (30 mL), heated to reflux for 6 h. Sampling point plate monitoring 2,5-dihydroxybenzaldehyde point basically completes the reaction, stop heating, and cool down to room temperature. Suction filtration, and the filtrate was concentrated under reduced pressure to dryness to obtain an oily substance. Add 30 mL of anhydrous ethanol to the oil, stir and heat to 45°C to dissolve, then cool to room temperature after dissolving, slowly add 120 mL of ice water, stir and crystallize for 0.5 to 1 h, filter with suction, and blow dry the filter cake to obtain 4.30 g of a yellow-brown solid.
[0057] S2. Liquid phase preparation and purification:
[0058] Take the yellow-brown solid, add acetonitrile ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com