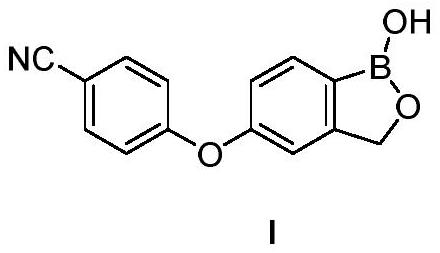
Preparation method of crisaborole
A technology of crisaborol and solvent, which is applied in the field of preparation of crisaborol, can solve problems such as cost increase, heavy metal pollution, and high cost, and achieve the effects of easy operation, high safety, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0045]The preparation method includes:
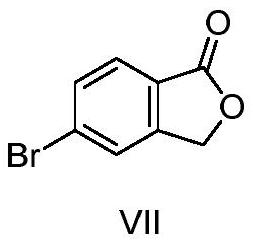
[0046](1) 5-bromophenate in formula VII
[0047]
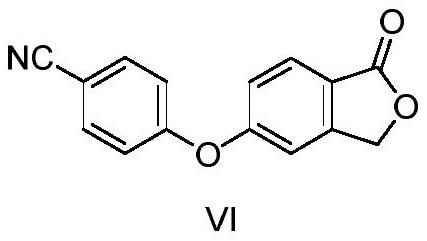
[0048]For the starting material, in the first solvent, the first base is etherified with an etherification reaction to obtain a compound of formula VI;
[0049]
[0050](2) The hydrolysis reaction of the formula Vi compound in the second solvent, the presence of the first acid or the second base is obtained by the formula V compound;
[0051]
[0052](3) Reacting the formula IV compound in the presence of the formula V compound in the third solvent, the alkali is reacted with the hydroxyl protective reagent;
[0053]
[0054]The PG is a hydroxy protecting group;
[0055](4) The compound of formula IV is carried out in a condensing agent under a fourth solvent, and the reaction is obtained by condensation of N-hydroxyphthalimide under the condensation agent;
[0056]
[0057](5) The compound of formula III is carried out under the fifth solvent, and the presence of the light source is irradiated, and the presence of the boron co...
Embodiment 1
[0109]Example 1: Preparation of a compound of formula VI
[0110]60.0 g (0.28 mol) 5-bromide (compound), 50.0 g (0.42 mol) 4-cyanophenol, 116.0 g (0.84 mol) potassium carbonate and 300 ml DMF were added to the reaction bottle, stirring and warming up to 95 ~ 100 ° C reacted for about 12 hours, cooling, adding water crystalline, filtration, drying, gauge white solid powder 58.2g, that is, the formula Vi compound 5- (4-nitrile phenoxy) phenylphaxire, yield 82.2% .
[0111]Nuclear magnetic data of the resulting solid:1H-NMR (400MHz, CDCL3: Δ7.97 (D, 1H), 7.74 (m, 2H), 7.22 (DD, 1H), 7.18 (m, 2H), 7.11 (D, 1H), 5.31 (S, 2H). ,
Embodiment 2
[0112]Example 2: Preparation of a compound of formula V
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com