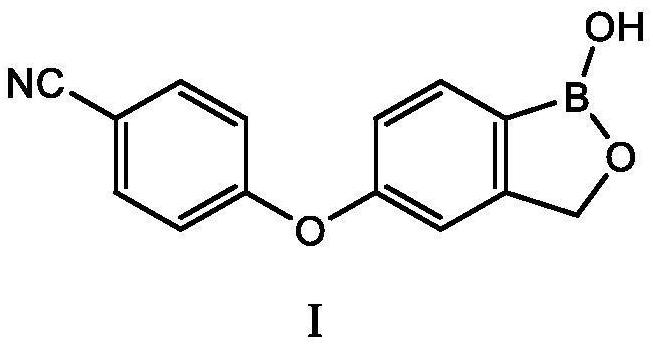
Preparation method of crisaborole
A technology of cleaborol and tolyl boronic acid, which is applied in the field of medicines for the treatment of skin diseases, can solve problems such as unsatisfactory yield and purity, and achieve the effects of novel synthesis route, mild reaction, and avoiding ultra-low temperature reaction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
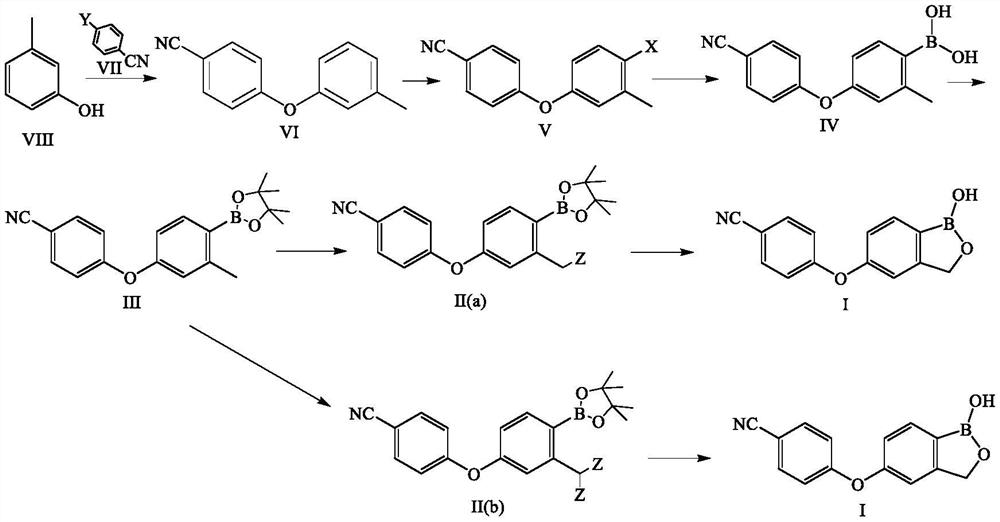
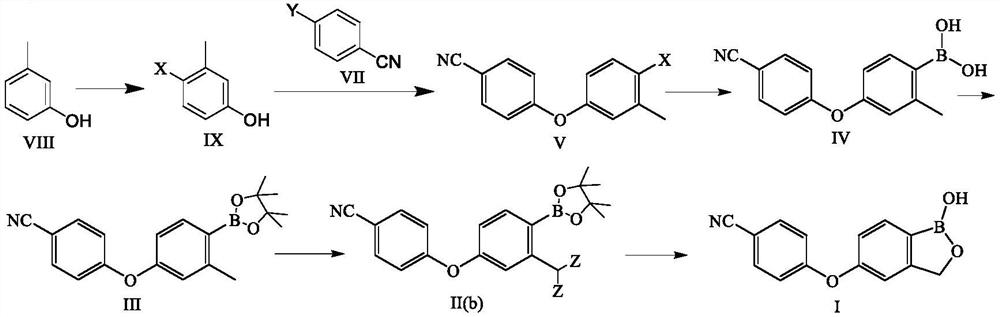
[0109] Embodiment 1: the preparation of 4-(3-methylphenoxy)benzonitrile of structural formula VI
[0110]
[0111] Put 35g of m-cresol (0.32mol), 46g of 4-fluorobenzonitrile (0.38mol), 350mL of DMF and 90g of potassium carbonate into a 1L reaction flask in sequence; raise the temperature to 120°C, react for 12 hours, then cool down to 25°C; 800mL of water, stirred for 1 hour, filtered; the filter cake was dissolved in 300mL of dichloromethane, concentrated under reduced pressure, the residue was recrystallized with n-heptane and ethyl acetate, filtered; dried to obtain 4-(3-methyl phenoxy)benzonitrile 57g, yield 85%, purity 99%.
[0112] Mass: m / z 210.1[M-H] + ;
[0113] 1H NMR (400MHz, CDCl 3 )δ7.63-7.53(m,2H),7.30-7.24(m,1H),7.03(d,J=7.6Hz,1H),7.01-6.96(m,2H),6.89-6.82(m,2H) ,2.36(s,3H).
Embodiment 2
[0114] Embodiment 2: the preparation of 4-(3-methylphenoxy)benzonitrile of structural formula VI
[0115]
[0116] Put 35g of m-cresol (0.32mol), 52g of 4-chlorobenzonitrile (0.38mol), 350mL of DMF, and 90g of potassium carbonate into a 1L reaction flask in sequence; heat up to 160°C, react for 18 hours, and cool down to 25°C; Add 800mL of water, stir for 1 hour, and filter; the filter cake is dissolved in 300mL of dichloromethane, concentrated under reduced pressure, and the residue is recrystallized with n-heptane and ethyl acetate, and filtered; dried to obtain 4-(3-methanol as a white solid) phenoxy) benzonitrile 54g, yield 80%, purity 99%.
[0117] Mass: m / z 210.1[M-H] + ;
[0118] 1H NMR (400MHz, CDCl 3 )δ7.63-7.53(m,2H),7.30-7.24(m,1H),7.03(d,J=7.6Hz,1H),7.01-6.96(m,2H),6.89-6.82(m,2H) ,2.36(s,3H).
Embodiment 3
[0119] Embodiment 3: Preparation of 4-(4-bromo-3-methylphenoxy)benzonitrile of structural formula V
[0120]
[0121] 63g of 4-(3-methylphenoxy)benzonitrile (0.3mol), 58g of N-bromosuccinimide (0.33mol), and 300mL of methanol were successively put into a 1L reaction flask; the temperature was raised to 50°C, and the reaction After 6 hours, cool down to 25°C; add the reaction solution to 300mL water, stir for 1 hour, and filter; dissolve the filter cake in 300mL of dichloromethane, concentrate under reduced pressure, recrystallize the residue with n-heptane and ethyl acetate, filter; dry 69 g of 4-(4-bromo-3-methylphenoxy)benzonitrile was obtained as a white solid, with a yield of 80% and a purity of 99%.
[0122] Mass: m / z 288.0[M-H]+ ;
[0123] 1H NMR (400MHz, CDCl 3 )δ7.61(d, J=7.2Hz, 2H), 7.54 (d, J=7.2Hz, 1H), 7.00(d, J=6.8Hz, 2H), 6.95(s, 1H), 6.64 (dd, J1=2.0Hz, J2=6.8Hz, 1H), 2.39(s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com