Erlotinib, and preparation method of new intermediate of erlotinib
A kind of technology of erlotinib and intermediate, applied in the field of drug development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
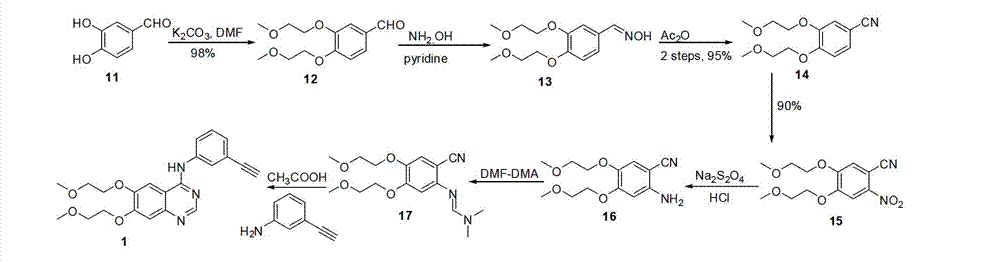
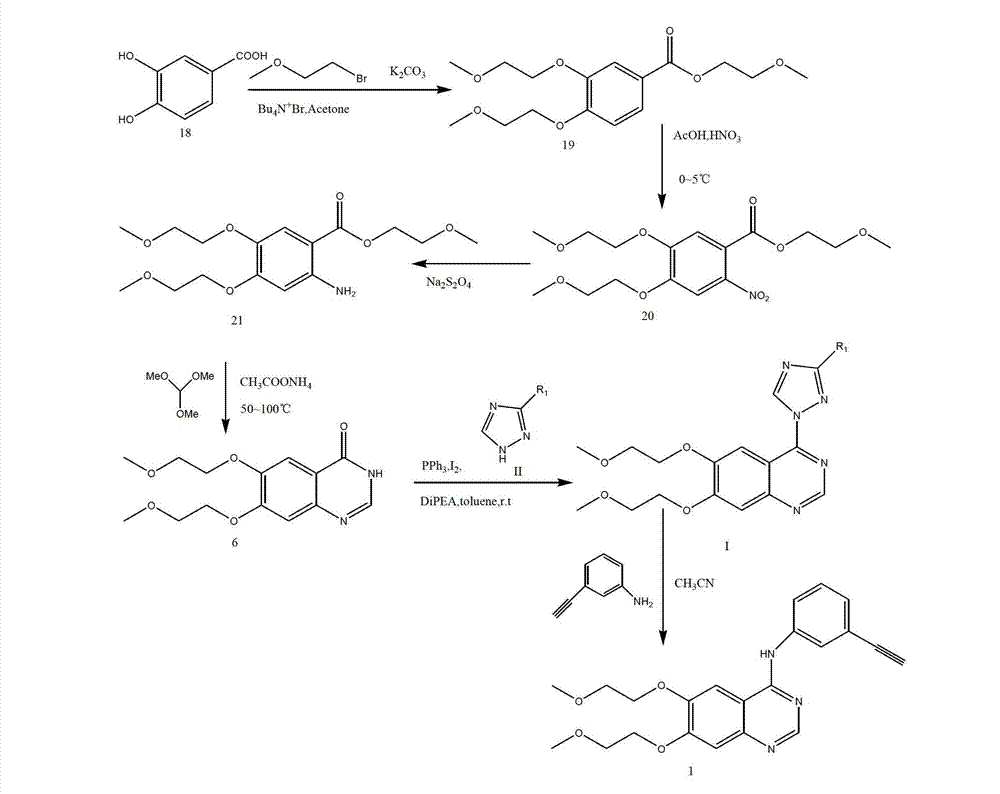
Image
Examples
Embodiment 1
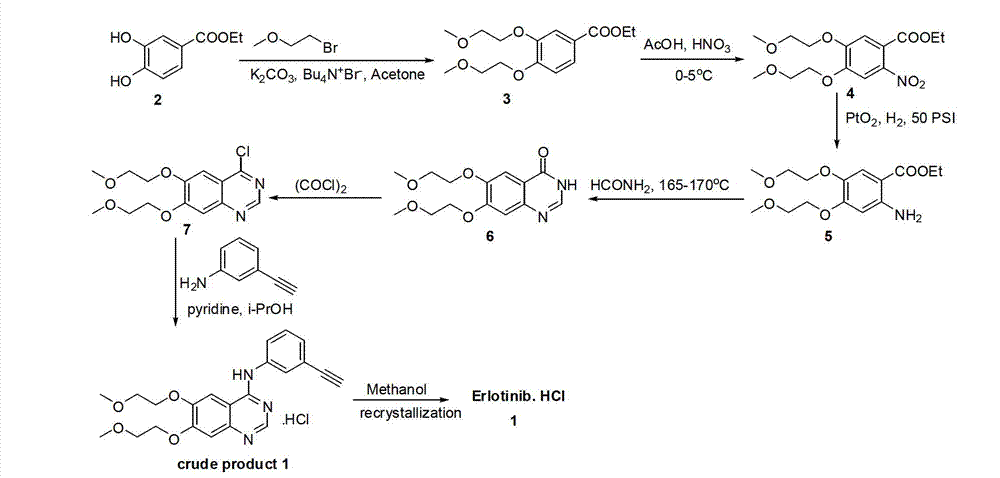
[0054] Example 1 3, the preparation of 4-bis-(2-methoxyethoxy)-benzoic acid (2-methoxyethyl ester)
[0055] In a 1L reaction flask, add 20g of 3,4-dihydroxybenzoic acid and 400mL of acetone, then add 30g of potassium carbonate, 1g of potassium iodide, and 30mL of 2-bromoethoxymethyl ether, heat to reflux for 12 hours, cool and filter, and the filtrate After concentration, 38.8 g of a light yellow solid was obtained, with a yield of 91.5%.
[0056] 1 H NMR (CDCl 3 , 400MHz) δ7.72(dd, J=8.4, 2.0Hz, 1H), 7.61(d, J=2.0Hz, 1H), 6.93(d, J=8.4Hz, 1H), 4.47-4.44(m, 2H ), 4.23-4.20(m, 4H), 3.83-3.80(m, 4H), 3.74-3.72(m, 2H), 3.47(s, 6H), 3.44(s, 3H).
[0057] ESI-MS m / z 328.6M + .
Embodiment 2
[0058] Example 2 Preparation of 2-nitro-4,5-bis-(2-methoxyethoxy)-benzoic acid (2-methoxyethyl ester)
[0059] In a 1L reaction flask, add 3,4-bis-(2-methoxyethoxy)-benzoic acid (2-methoxyethyl ester) 38.5g and 120mL acetic acid, keep the temperature below 5°C and add 45% HNO3 (20mL) was reacted for 30 minutes, then 70% H2SO4 (40mL) was added dropwise, and the solution was reacted at room temperature for 2 hours. Add ice water and 50% NaOH to make it alkaline, extract with ethyl acetate, filter, and concentrate the filtrate to obtain 32.0 g of a light yellow solid with a yield of 89%.
[0060] 1 H NMR (CDCl 3 , 400MHz) δ7.54(s, 1H), 7.14(s, 1H), 4.47(t, J=5.2Hz, 1H), 4.26-4.24(m, 4H), 3.82-3.79(m, 4H), 3.71 (t, J=5.2Hz, 2H), 3.46(s, 6H), 3.40(s, 3H).
[0061] ESI-MS m / z 373.6M + .
Embodiment 3
[0062] Example 3 Preparation of 2-amino-4,5-bis-(2-methoxyethoxy)-benzoic acid (2-methoxyethyl ester)
[0063] In a 500mL reaction flask, add 2-nitro-4,5-bis-(2-methoxyethoxy)-benzoic acid (2-methoxyethyl ester) 15g and 150mL methanol, add 10% Pd / C, hydrogenation under normal pressure for 6 hours, filtered, and the filtrate was concentrated to obtain 14.3 g of a brown solid, with a yield of 99%.
[0064] ESI-MS m / z 343.6M + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com