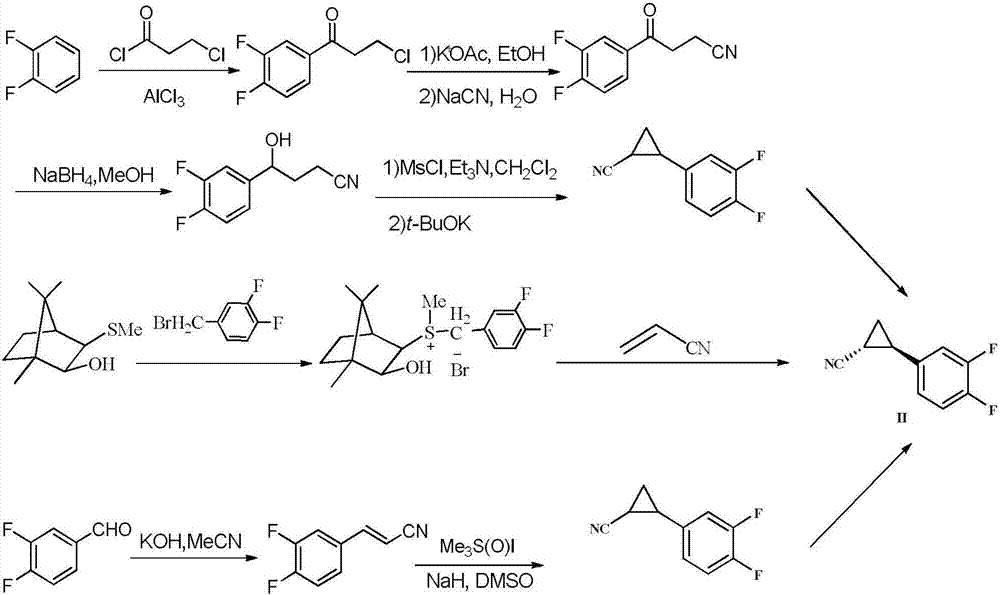
Novel synthesis method of ticagrelor intermediate (1R,2R)-2-(3,4-difluorophenyl) cyclopropane nitrile
A technology of difluorophenyl and ticagrelor, which is applied in the fields of organic chemistry methods, chemical instruments and methods, preparation of sugar derivatives, etc. It can solve the problems of heavy environmental pollution, poor atom economy, difficult recycling and reuse in the production process, etc. , to achieve the effect of improving the production environment, mild reaction conditions, and easy separation and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-2
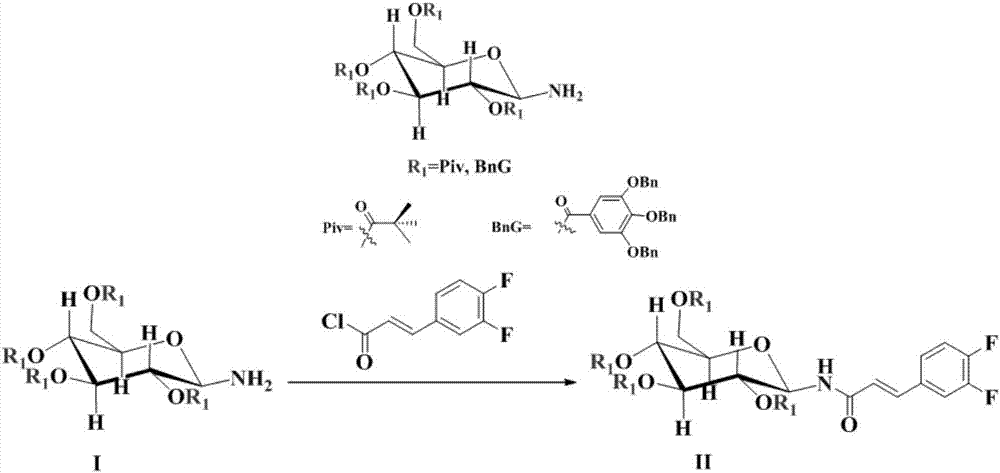
[0036] Example 1-2. N-[2,3,4,6-Tetra-O-(3,4,5-tribenzyloxy)benzoyl-β-D-glucopyranosyl]-(2E) Synthesis of -3-(3,4-difluorophenyl)acrylamide
[0037] Add N-[2,3,4,6-tetra-O-(3,4,5-tribenzyloxy)benzoyl-β-D-glucopyranosyl]amine in a 500mL four-necked flask I (36.1g, 0.02mol), toluene (200mL) and triethylamine (3.03g, 0.03mol), placed at 0℃, slowly add (2E)-3-(3,4-difluorophenyl) dropwise ) Acrylic acid chloride toluene solution (4.44g(2E)-3-(3,4-difluorophenyl)acrylic acid chloride dissolved in 100 mL of toluene), after dripping, the temperature is raised to 65℃ and the reaction is kept warm. The reaction is detected by TLC and the reaction is over After cooling to room temperature, use 5% NaCl solution and 6% NaHCO 3 The solution was washed with purified water and distilled under reduced pressure to obtain 37.64 g of pale yellow solid. The product yield is 95.5%, and the HPLC content is 99.0%. Example 1-3. N-(2,3,4,6-Tetra-O-pivaloyl-β-D-glucopyranosyl)-(2E)-3-(3,4-difluorophenyl...
Embodiment 1-3
[0038] Add N-[2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyl]amine I (10.3g, 0.02mol), toluene (200mL) to a 500mL four-necked flask. ) And pyridine (2.37g, 0.03mol), placed at 0℃, slowly add (2E)-3-(3,4-difluorophenyl)acryloyl chloride toluene solution (4.44g(2E)-3- (3,4-Difluorophenyl)acryloyl chloride dissolved in 100 mL of toluene). After the addition, the temperature is raised to 65°C and the reaction is kept warm. The reaction is detected by TLC. After the reaction is completed, it is cooled to room temperature, and 5% NaCl solution is used. 6% NaHCO 3 The solution was washed with purified water and distilled under reduced pressure to obtain 13.35 g of off-white solid. The product yield is 98.0%, and the HPLC content is 99.6%.
Embodiment 1-4
[0039] Example 1-4. N-(2,3,4,6-Tetra-O-pivaloyl-β-D-glucopyranosyl)-(2E)-3-(3,4-difluorophenyl ) Synthesis of acrylamide
[0040] Add N-[2,3,4,6-tetra-O-pivaloyl-β-D-glucopyranosyl]amine I (10.3g, 0.02mol), toluene (200mL) to a 500mL four-necked flask. ) And pyridine (2.84g, 0.036mol), placed at 0℃, slowly add dropwise (2E)-3-(3,4-difluorophenyl)acryloyl chloride toluene solution (0.024mol(2E)-3- (3,4-Difluorophenyl)acryloyl chloride dissolved in 100 mL of toluene). After the addition, the temperature is raised to 60°C and the reaction is kept warm. The reaction is detected by TLC. After the reaction is completed, it is cooled to room temperature and 5% NaCl solution is used. 6% NaHCO 3 The solution was washed with purified water and distilled under reduced pressure to obtain 13.28 g of off-white solid. The product yield is 97.5%, and the HPLC content is 99.5%.
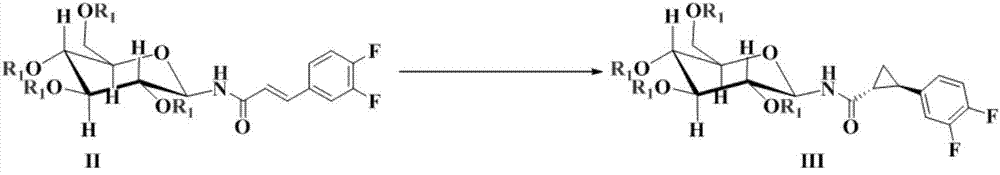
[0041] Then, the glucosyl α, β-unsaturated amide compound II prepared in any of the above embodiments 1-1 to 1-4 is u...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com