Compound and preparation method and application thereof
A compound, the technology of boron trifluoride ether, is applied in the field of preparation of the impurities of Liejing drugs, and achieves the effects of single structure, few by-products, and avoiding post-processing difficulties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
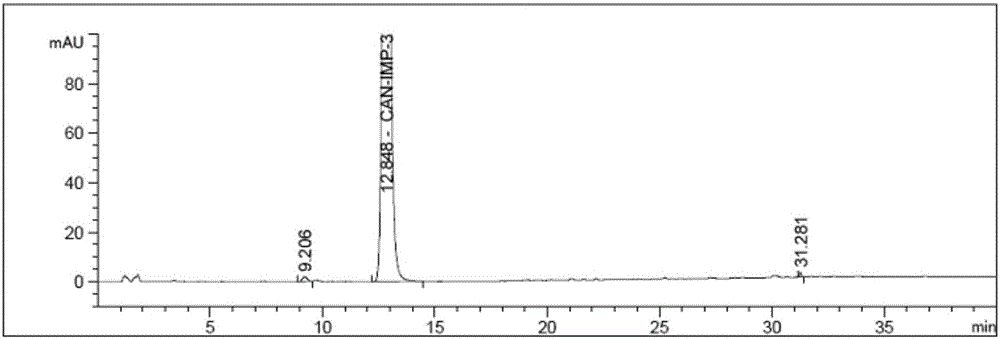
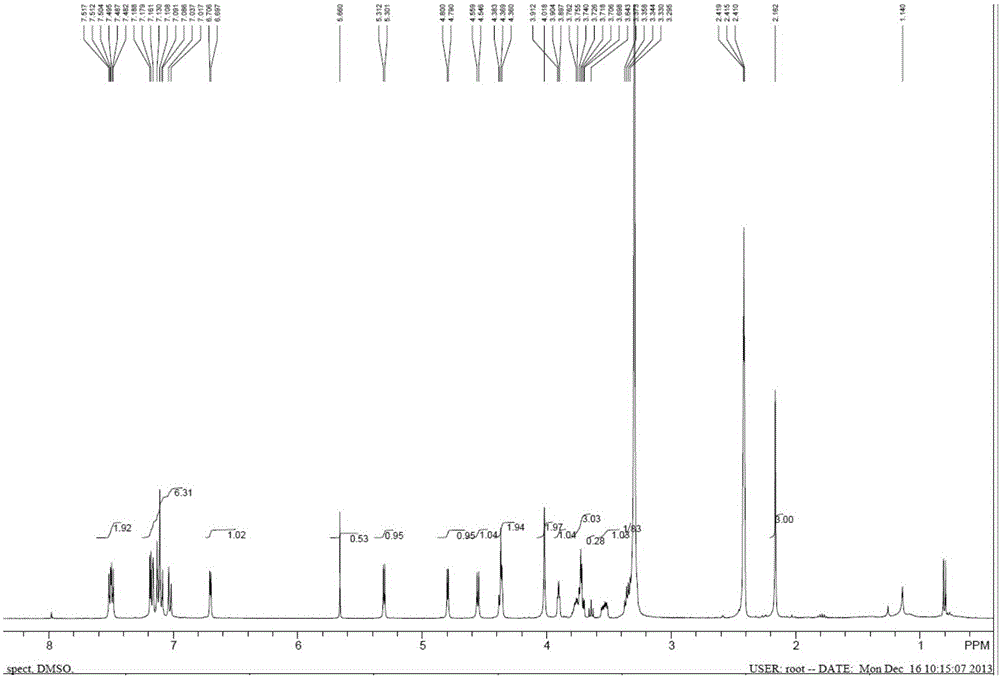
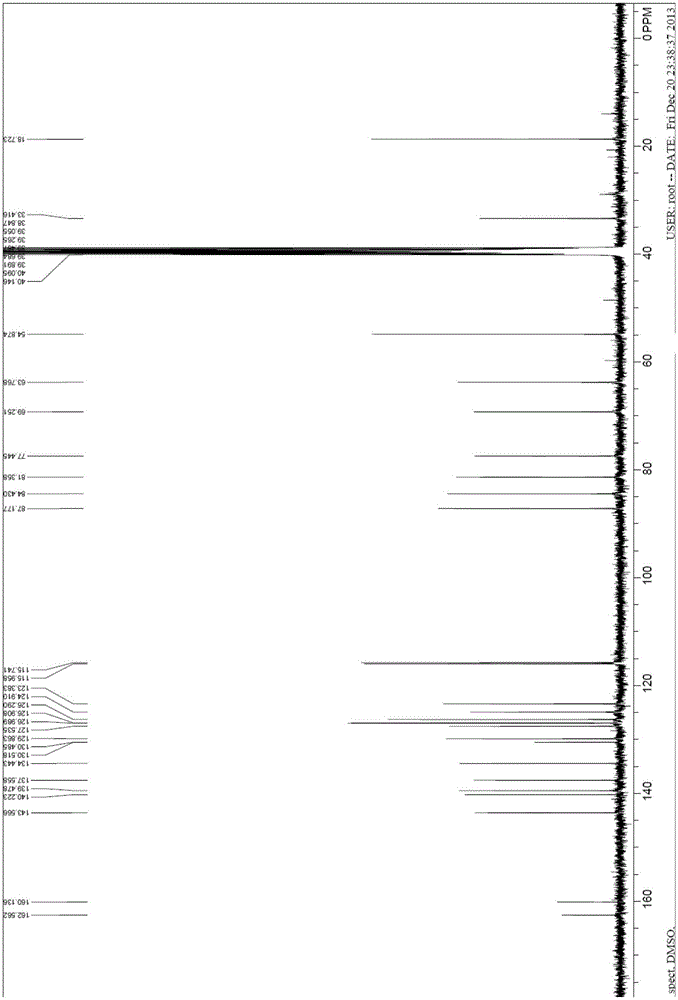
Image
Examples
Embodiment 1
[0056] Compound shown in embodiment 1 synthetic formula 2
[0057] The steps of the compound shown in the synthetic formula 2 are as follows:
[0058] 1. Add a few drops of concentrated sulfuric acid dropwise to a methanol solution in which the compound 3,5,6-tribenzyl-D-glucofuranose (50g, 110mmol) is dissolved, stir and heat to 50 to 55°C for 2 hours, and monitor the raw material by TLC The response is complete.
[0059] 2. Concentrate the solvent under reduced pressure to remove water, and dissolve the residue in 300ml ethyl acetate
[0060] 3. Add 100ml of water to the product in step 2, and adjust the pH value to close to 8 with solid sodium bicarbonate.
[0061] 4. The product in step 3 was separated, washed with water and brine respectively, dried over anhydrous sodium sulfate, and concentrated to obtain about 51 g (109.8 mmol) of a colorless oil, which could be directly used in the next reaction.
Embodiment 2
[0062] Compound shown in embodiment 2 synthetic formula 3
[0063] The steps of the compound shown in the synthetic formula 3 are as follows:
[0064] 1. Weigh 34 g (about 73 mmol) of the oil obtained in Example 1, and dissolve it in N,N-dimethylformamide (340 ml).
[0065] 2. Cool the solution obtained in step 1 to 0-5° C. with an ice-water bath, and add 60% sodium hydride (3.07 g, 77 mmol, 1.05 equivalents) in batches.
[0066] 3. Stir at 0-5°C for 30 minutes. A N,N-dimethylformamide solution containing benzyl bromide (12.5g, 74.5mmol) was slowly added dropwise, and the internal temperature was controlled at 0-5°C during the dropwise addition.
[0067] 4. React the solution obtained in step 3 at room temperature for 2 hours.
[0068] 5. The reaction solution obtained in step 4 was poured into water, extracted with ethyl acetate, washed with water, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 35 g (63.1 mmol) of a light yello...
Embodiment 3
[0069] Compound shown in embodiment 3 synthetic formula 4
[0070] The steps of the compound shown in the synthetic formula 4 are as follows:
[0071] 1. Weigh 21 g (about 37.9 mmol) of the oil obtained in Example 2, dissolve it in dioxane, and add 2N hydrochloric acid aqueous solution under stirring.
[0072] 2. Stir the solution obtained in step 1, and raise the temperature to 70-80° C. to react for 16 hours, and monitor the reaction to the end by thin-layer chromatography.
[0073] 3. Pour the reaction solution into water, and adjust the pH value to close to 8 with solid sodium bicarbonate.
[0074] 4. Extract the solution obtained in step 3 with ethyl acetate, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, and concentrate to obtain a light yellow oil, which is the crude product of the compound shown in formula 4.
[0075] 5. The crude product obtained by the above treatment was purified by column chromatography to obtain 14 g of a colorles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com