Method for synthesizing crisaborole intermediate by using microchannel reactor
A technology of microchannel reactors and channel reactors, applied in chemical instruments and methods, chemical/physical/physical chemical reactors, compounds containing elements of Group 3/13 of the periodic table, etc., to achieve increased purity and yield, The effect of reducing the content of impurities and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
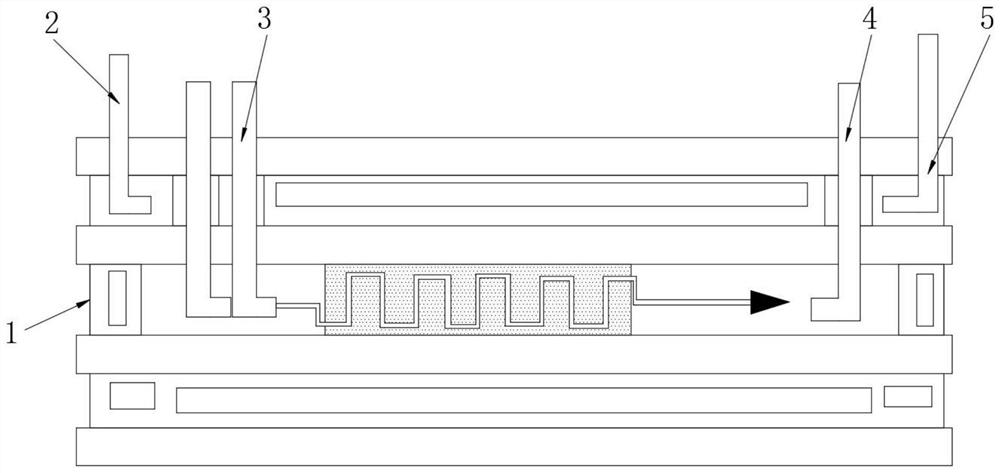
Image
Examples
Embodiment 1
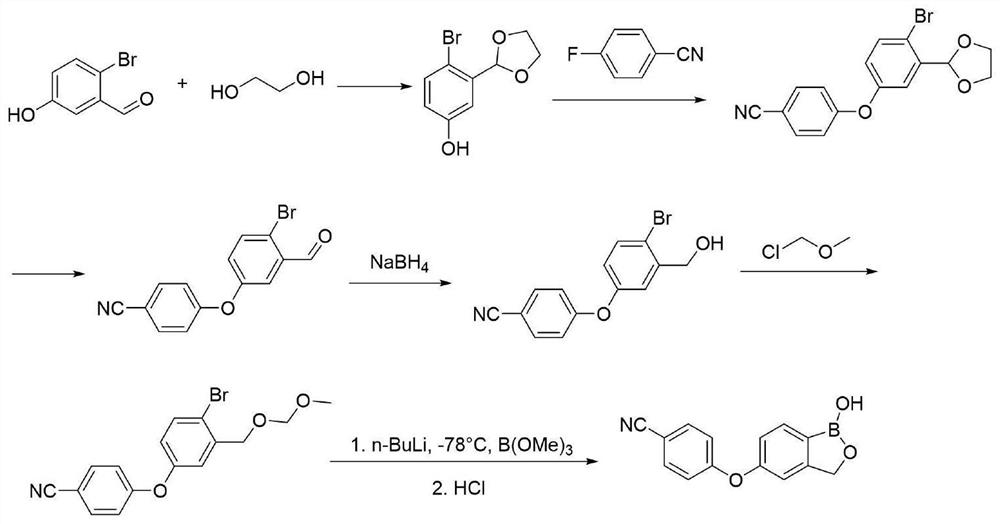
[0041] A method for synthesizing a crisborole intermediate using a microchannel reactor, specifically comprising the following steps:
[0042] Step 1, dissolving the intermediate a in the organic solution b, mixing evenly, as the material 1, transporting the material 1 to the pre-cooling module in the microchannel reactor through the plunger pump for mixing and pre-cooling;
[0043] Step 2, the reaction alkali solution is used as material 2, and the material 2 is transported to the precooling module in the microchannel reactor by a plunger pump for mixing and precooling;
[0044]Step 3, the precooled material 1 and material 2 in the step 1 and step 2 are delivered to the reaction module group of the microchannel reactor for reaction, and the material 3 is obtained;
[0045] Step 4, dissolving the boric acid ester in the organic solvent c, mixing evenly, as the material 4, transporting the material 4 to the pre-cooling module in the microchannel reactor through the plunger pump...
Embodiment 2
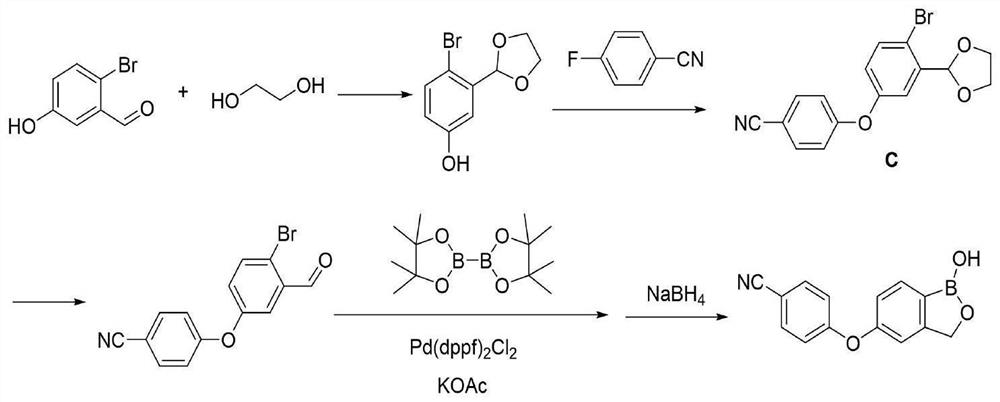
[0058] A method for synthesizing a crisborole intermediate using a microchannel reactor, specifically comprising the following steps:
[0059] Step 1, dissolving the intermediate a in the organic solution b, mixing evenly, as the material 1, transporting the material 1 to the pre-cooling module in the microchannel reactor through the plunger pump for mixing and pre-cooling;
[0060] Step 2, the reaction alkali solution is used as material 2, and the material 2 is transported to the precooling module in the microchannel reactor by a plunger pump for mixing and precooling;
[0061] Step 3, the precooled material 1 and material 2 in the step 1 and step 2 are delivered to the reaction module group of the microchannel reactor for reaction, and the material 3 is obtained;
[0062] Step 4, dissolving the boric acid ester in the organic solvent c, mixing evenly, as the material 4, transporting the material 4 to the pre-cooling module in the microchannel reactor through the plunger pum...
Embodiment 3
[0075] A method for synthesizing a crisborole intermediate using a microchannel reactor, specifically comprising the following steps:
[0076] Step 1, dissolving the intermediate a in the organic solution b, mixing evenly, as the material 1, transporting the material 1 to the pre-cooling module in the microchannel reactor through the plunger pump for mixing and pre-cooling;
[0077] Step 2, the reaction alkali solution is used as material 2, and the material 2 is transported to the precooling module in the microchannel reactor by a plunger pump for mixing and precooling;
[0078] Step 3, the precooled material 1 and material 2 in the step 1 and step 2 are delivered to the reaction module group of the microchannel reactor for reaction, and the material 3 is obtained;
[0079] Step 4, dissolving the boric acid ester in the organic solvent c, mixing evenly, as the material 4, transporting the material 4 to the pre-cooling module in the microchannel reactor through the plunger pum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com