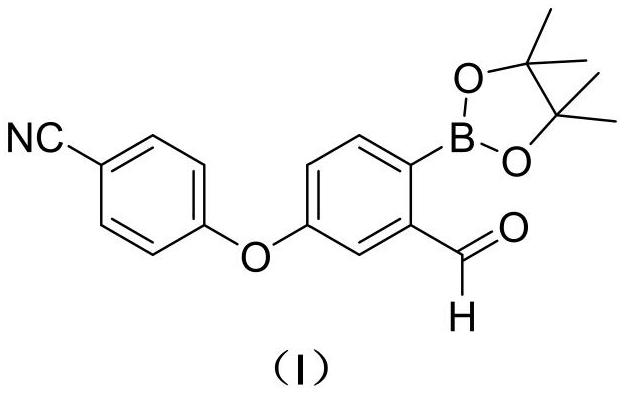
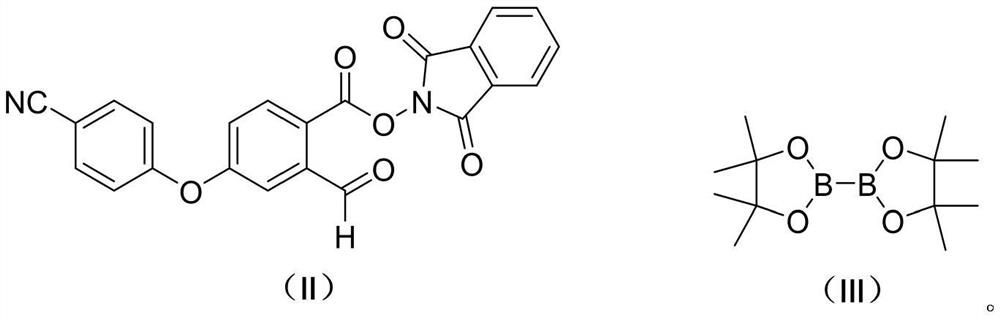
Preparation method of 2-formyl-4-(4-cyanophenoxy) phenylboronic acid pinacol ester
A pinacol ester and formyl technology is applied in the field of intermediates for preparing criborole, can solve the problems of many side reactions, high cost, serious environmental pollution and the like, achieves improved yield and purity, reduced side reactions, Environmentally friendly effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of Compound Ⅵ-a
[0036]
[0037] 1L three-neck flask was added 300mL of methylene chloride and N- hydroxy-phthalimide (17.9g, 0.11mol), stirred clear solution, was added 2-formyl-4-chlorobenzoic acid (22.2g, 0.12mol) and 4-dimethylaminopyridine (0.61g, 5mmol), was added dropwise at room temperature, dicyclohexyl carbodiimide (24.8g, 0.12mol) in dichloromethane (100mL) and stirring was continued for 3h after the addition was complete. Filtered, the white precipitate was filtered off, the filtrate was concentrated under reduced pressure to dryness compound Ⅵ-a, was used without further purification in the next reaction.
Embodiment 2
[0039] Preparation of Compound Ⅱ
[0040]
[0041] Acetone compound obtained in Example 1 Ⅵ-a was dissolved in acetone (200mL) was added anhydrous potassium carbonate (19.4g, 0.14mol), a solution of 4-hydroxybenzonitrile at room temperature (14.3g, 0.12mol) in (60 mL) was, after the dropwise addition was stirred for 6h. 650mL of cold water was added dropwise, the temperature controlled at 20 ~ 30 ℃, After completion of the dropwise addition stirring was continued for 2h, filtered and the filter cake was rinsed with 150mL of water three times. The resulting solid was refluxed in ethyl acetate (90 mL) IH beating, cooling, stirring was continued at 0 ~ 5 ℃ 2h, filtered and the filter cake was dried under reduced pressure to give 36.7g compound Ⅱ. Yield 80.9% (Ⅴ compound basis).
[0042] MS (ESI, m / z): 413.1 (M + 1) +
[0043] 1 H-NMR (400MHz, CDCl3) δ (ppm): 7.87-7.91 (m, 2H), 7.84-7.88 (m, 3H), 7.59-7.61 (m, 2H), 7.08 (m, 1H), 7.00-7.03 (m, 2H), 6.97 (m, 1H).
Embodiment 3
[0045] Preparation of compound Ⅰ
[0046]
[0047] 500mL three-neck flask was added compound Ⅱ (20.6g, 50mmol) and the compound Ⅲ (25.4g, 100mmol), was added after the nitrogen thrice with ethyl acetate (250 mL) and isonicotinic acid tert-butyl ester (1.79g, 10mmol), nitrogen heated to 70 ℃ ~ 75 ℃ reaction was 17h. Cooling, concentrated under reduced pressure, the residue was added 100mL of n-heptane and 0.8g of activated carbon, heated to reflux for 10min, filtered while hot and the filtrate cooled crystallization, stirred for 3h, filtered off with suction, the filter cake was dried to give 14.8g compound Ⅰ 0 ℃ ~ 5 ℃ a yield of 84.8%.
[0048] MS (ESI, m / z): 350.1 (M + 1) +
[0049] 1 H-NMR (400MHz, CDCl3) δ (ppm): 7.85 (m, 1H), 7.61 (m, 2H), 7.08 (m, 1H), 7.00 ~ 7.03 (m, 2H), 6.97 (m, 1H), 1.34 (s, 12H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com