Preparation method of crisaborole
A technology of crisborol and borohydride, which is applied in the field of preparation of crisborol, can solve the problems of harsh reaction conditions of butyllithium or tert-butyllithium, expensive organic palladium catalyst, high process cost, etc. The effect of cheap price, low price and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
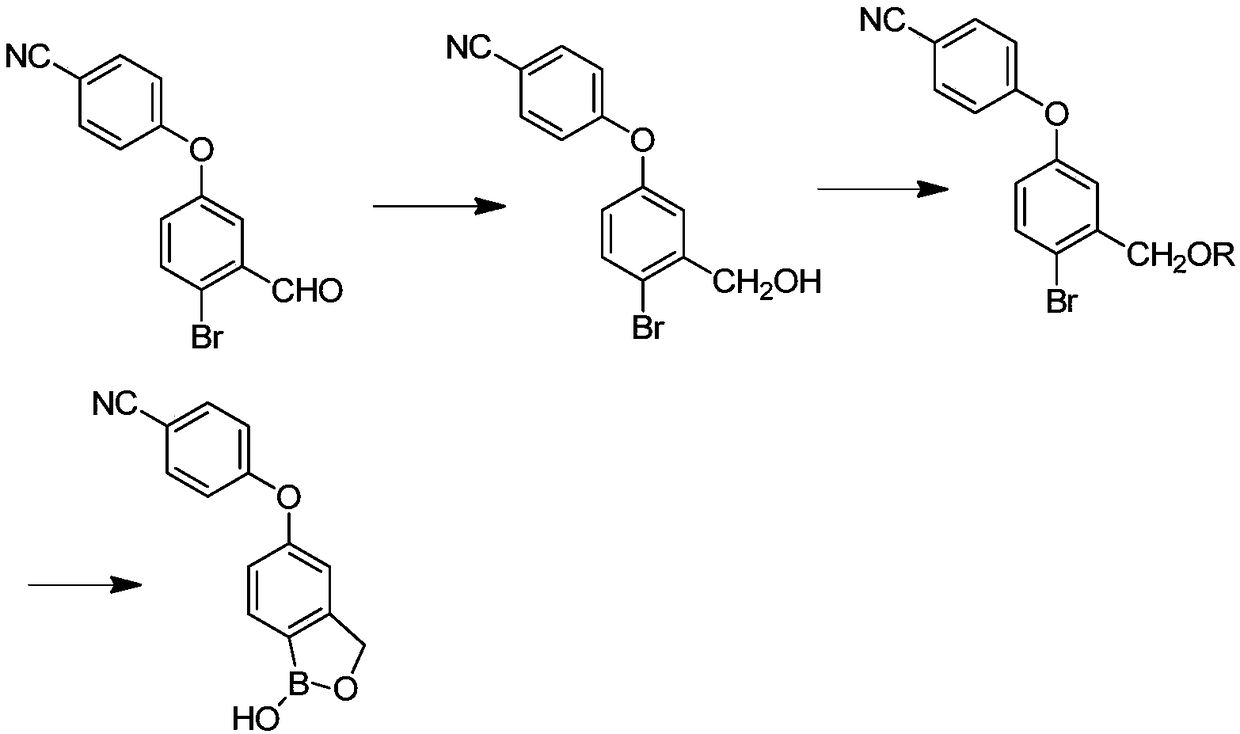
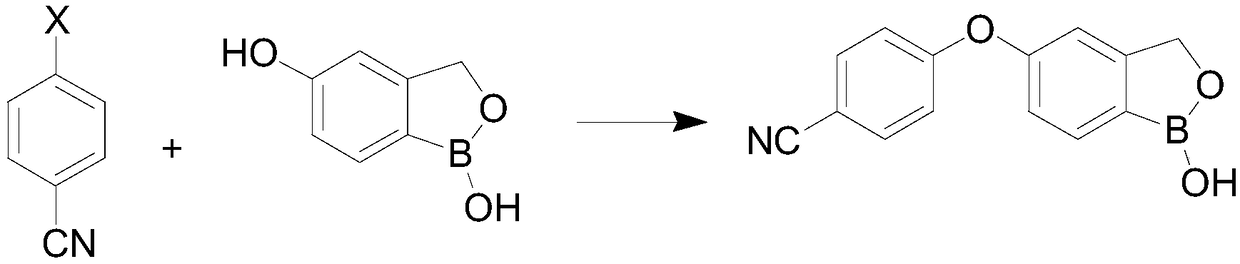
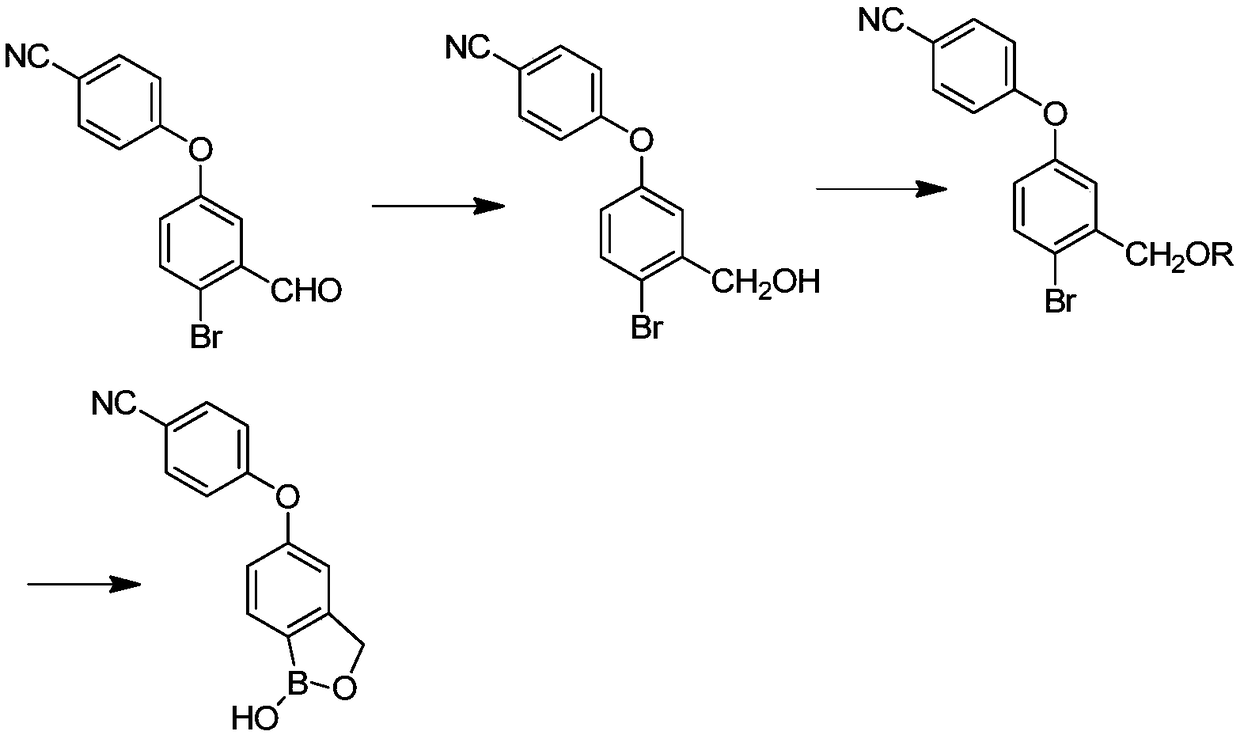
[0023] Such as image 3 As shown, the invention provides a kind of preparation method of crisborole, and this preparation method comprises the steps:
[0024] (1) In the presence of the first solvent, the compound shown in formula I is contacted with an alkali metal borohydride to obtain the compound shown in formula II;
[0025]
[0026] (2) In the presence of a second organic solvent and a base, the compound shown in formula II and compound a are subjected to a contact reaction to obtain a compound shown in formula III; or in the presence of a second organic solvent and a catalyst, the The compound shown in the formula II and dihydropyran are subjected to a contact reaction to obtain the compound shown in the formula III; wherein, the compound a is trimethylchlorosilane, tert-butyldimethylsilyl chloride or chloromethyl methyl ether ;
[0027]
[0028] Wherein, R is trimethylsilyl, tert-butyldimethylsilyl, methyl methyl ether or tetrahydropyranyl;
[0029] (3) In the...
Embodiment
[0039] This embodiment provides a preparation method of crisborole, the preparation method comprising the following steps:
[0040] (1) Preparation of 4-[(4-bromo-3-hydroxymethyl)phenoxy)]benzonitrile (compound shown in formula II)
[0041] With 30.2g (0.1mol) 4-[(4-bromo-3-formyl) phenoxy group)] benzonitrile (compound shown in formula I), 400ml methyl alcohol join in the reactor, add 1.89g ( 0.1mol) sodium borohydride, after adding, continue to stir at 25°C for 4h until the reaction of the raw materials is complete, recover methanol, add 1000ml of water for beating, filter, and dry to obtain a solid of 27.8% with a yield of 92%;
[0042] (2) Preparation of [5-(4-cyanophenoxy)-2-bromophenyl-1-methoxy]-trimethylsilane (compound shown in formula III)
[0043] 30.2g (0.1mol) 4-[(4-bromo-3-hydroxymethyl)phenoxy)]benzonitrile, 300ml dichloromethane, 30.3g triethylamine (0.3mol) were added to the reactor, Cool to 0-10°C, add 12.96g of trimethylchlorosilane dropwise, control the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com