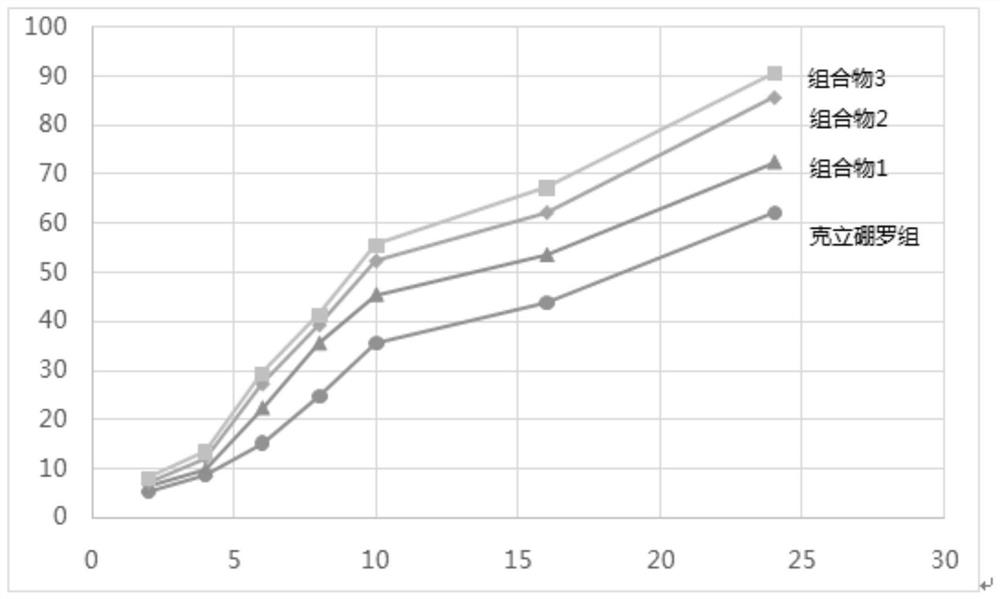
Pharmaceutical composition, pharmaceutical preparation and application of pharmaceutical composition and pharmaceutical preparation
A technology for pharmaceutical preparations and compositions, which is applied to pharmaceutical compositions, pharmaceutical preparations and their application fields, can solve the problems of strong drug resistance, easy recurrence, and large side effects, achieve good skin penetration, good anti-inflammatory effect, and promote release effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
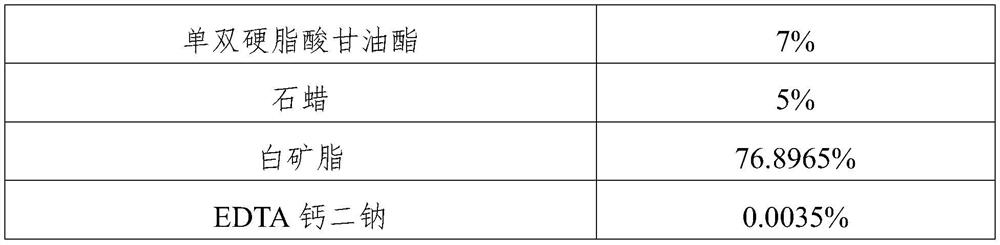
[0026] (1) Preparation of the oil phase: Add white petrolatum, paraffin, glyceryl mono- and distearate into the container, heat to 70-80°C to melt under stirring, then add butylated hydroxytoluene, stir to dissolve, and cool to 40-46°C to obtain an oil phase;
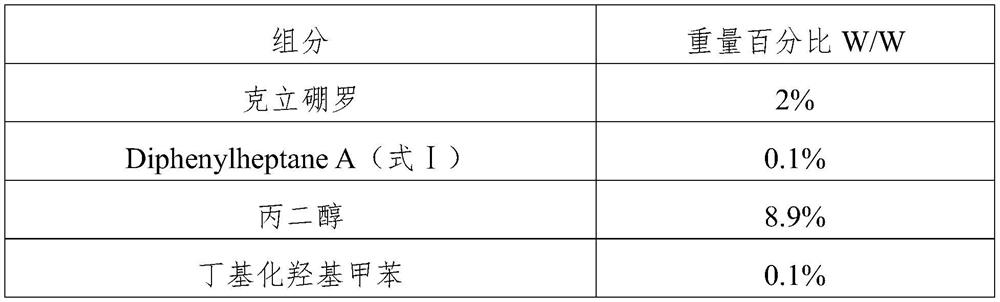
[0027] (2) Preparation of the solvent phase: add criborole, Diphenylheptane A, propylene glycol and calcium disodium EDTA into another mixing container, and heat to 40-46° C. under stirring to obtain the solvent phase;
[0028] (3) Emulsification: Add the solvent phase to the oil phase, keep the temperature at 40-46°C for 20 minutes, and cool to 25°C under stirring to obtain a uniform ointment.
[0029] The third aspect of the present invention provides the application of the above pharmaceutical composition in the preparation of anti-inflammatory drugs.
[0030] The fourth aspect of the present invention provides the application of the above-mentioned pharmaceutical composition in the preparation of a medicine for tre...
Embodiment 1
[0035] The present embodiment provides a kind of medicinal ointment, and the specific formula is shown in Table 1, and the specific preparation method is as follows:
[0036] (1) Preparation of the oil phase: Add white petrolatum, paraffin, glyceryl mono- and distearate into the container, heat to 75°C for melting under stirring, then add butylated hydroxytoluene, stir to dissolve, and cool to 45°C ℃, to obtain the oil phase;
[0037] (2) Preparation of the solvent phase: add crisborole, Diphenylheptane A, propylene glycol and calcium disodium EDTA into another mixing vessel, and heat to 45° C. under stirring to obtain the solvent phase;
[0038] (3) Emulsification: Add the solvent phase to the oil phase, keep the temperature at 45°C for 20 minutes, and cool to 25°C under stirring to obtain a uniform ointment.
[0039] Table 1
[0040]
[0041]
Embodiment 2
[0043] This embodiment provides a medicinal ointment, the specific formula is shown in Table 2, and the specific preparation method is the same as that of Embodiment 1.
[0044] Table 2
[0045] components Weight percentW / W Criborole 2% Diphenylheptane A (Formula Ⅰ) 0.2% Propylene Glycol 8.8% Butylated hydroxytoluene 0.1% Glyceryl monostearate 7% paraffin 5% white petrolatum 76.8965% Calcium disodium EDTA 0.0035%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com