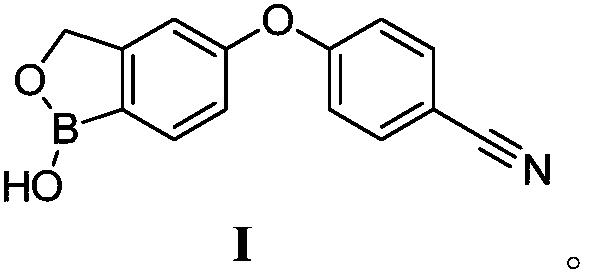
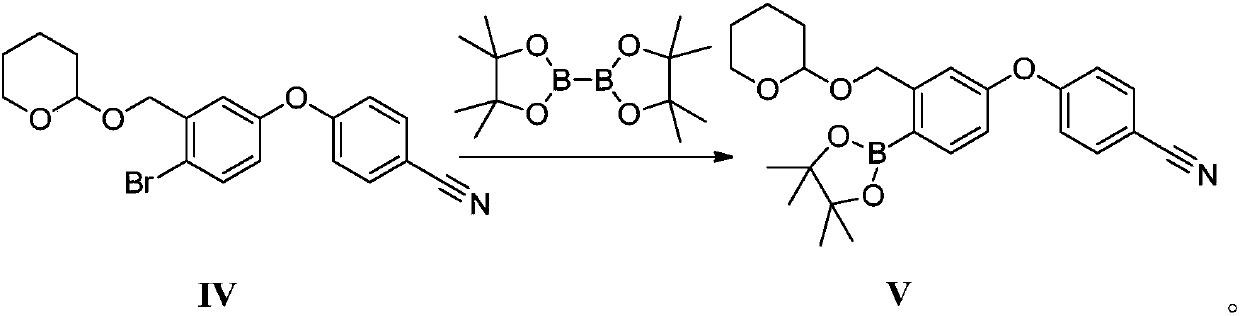
Method for preparing crisaborole
A technology of cressaboron and pinacol biboronate is applied in the field of preparation of cresaborate, which can solve the problems of difficult purification of products, low total yield, complicated steps and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
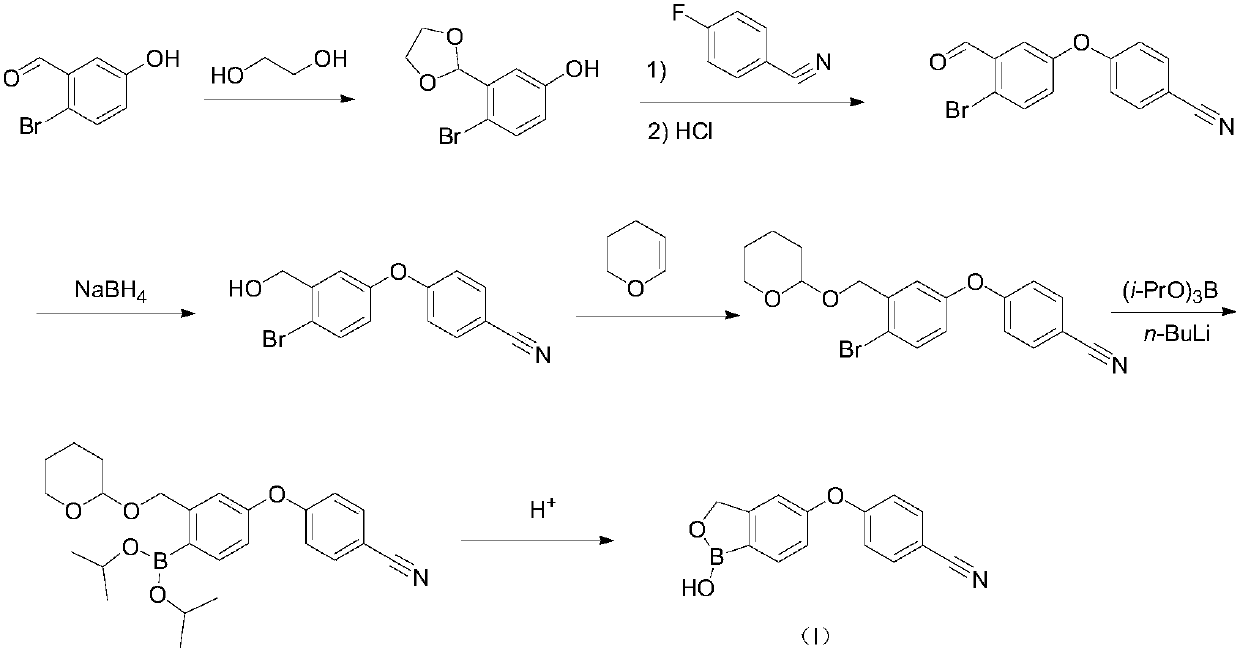
[0056] For the preparation of Intermediate III, the reaction formula is as follows:
[0057]
[0058] Add 2-bromo-5-hydroxybenzyl alcohol II (374g, 1.84mol), N,N-dimethylformamide (2.2L), potassium carbonate (509g, 2mol) and p-fluorobenzonitrile ( 223.1g, 1.84mol), after the addition, the reaction system was heated to 80-90°C for reaction, TLC monitored until the reaction was complete, and the system was slowly added to water (4.4L) for quenching.
[0059] Filtration and drying to obtain an off-white solid, ie Intermediate III, with an HPLC purity of 96.5% and a yield of 95.7%; 1 HNMR(300MHz, CDCl 3 )δ(ppm): 4.75(s, 2H), 6.88(dd, J=8.5, 2.9Hz, 1H), 7.02(d, J=8.8Hz, 1H), 7.26(d, J=2.6Hz, 1H) , 7.56 (d, J=8.5 Hz, 1H), 7.62 (d, J=8.8 Hz, 2H).
Embodiment 2
[0061] For the preparation of Intermediate III, the reaction formula is as follows:
[0062]
[0063] Add 2-bromo-5-hydroxybenzyl alcohol II (20.3g, 0.1mol), N,N-dimethylformamide (50mL), potassium carbonate (27.6g, 0.2mol) and p-fluorobenzene to a three-necked flask in sequence Nitrile (14.5g, 0.12mol), after the addition, the reaction system was heated to 80-90°C for reaction, TLC monitored until the reaction was complete, and the system was slowly added to water (250mL) for quenching.
[0064] After filtration and drying, an off-white solid, ie Intermediate III, was obtained with an HPLC purity of 93.5% and a yield of 96.2%.
Embodiment 3
[0066] For the preparation of Intermediate IV, the reaction formula is as follows:
[0067]
[0068] Intermediate III (30.4g, 0.1mol), dichloromethane (300ml), 2,3-dihydropyran (16.8g, 0.2mol) and p-toluenesulfonic acid monohydrate (1.9g, 0.01 mol), stirring overnight at room temperature.
[0069] Add 100ml of 1% ammonia water to the system, stir to separate the liquids, dry the organic phase with anhydrous sodium sulfate, filter, and concentrate to obtain a yellow oil. Add petroleum ether to crystallize to obtain an off-white solid, ie Intermediate IV, with a purity of 98.7% by HPLC , The yield is 88%; 1 HNMR(300MHz, CDCl 3 )δ(ppm) 1.64~1.86(m, 6H), 3.55(m, 1H), 3.88(m, 1H), 4.53(d, J=10.5Hz, 1H), 4.77(d, J=10.2, 2.7Hz , 2H), 6.84 (dd, J = 8.7, 4.2 Hz, 1H), 7.01 (dd, J = 6.6, 3.6 Hz, 2H), 7.27 (d, J = 2.4 Hz, 1H), 7.54 (d, J = 6.3 Hz, 1H), 7.60 (dd, J=6.6, 3.6 Hz, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com