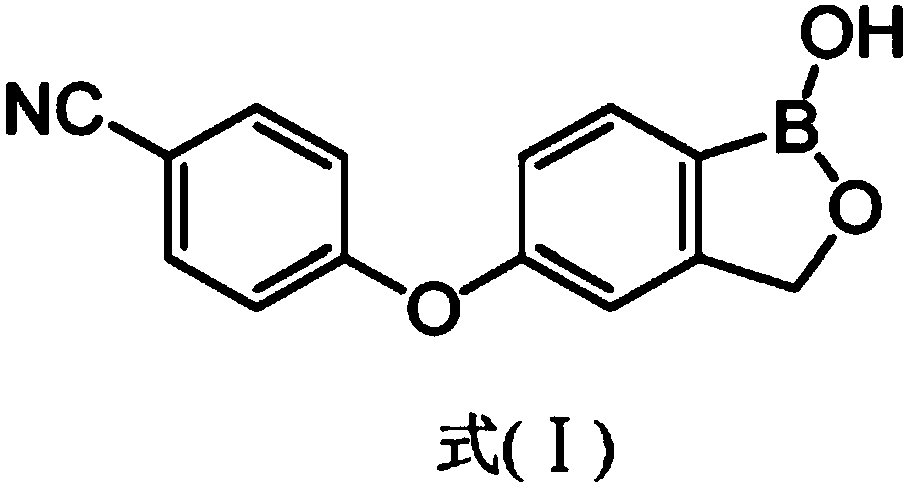
Preparation method of skin-touch crisaborole slow-release film
A technology of crisborole and slow-release membrane, which is applied in the field of biomedical materials, can solve the problems of not being able to retain for a long time and being easily wiped, and achieve the effect of enhancing drug penetration and adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A. Membrane matrix preparation: Spandex and silk are used as the matrix material to weave the matrix, and the moisture-discharging strips are arranged at intervals on the matrix. The moisture-relieving strips are made of antibacterial yarn and acrylic fiber, and the interval width is 2-3mm. The width of the moisture strip is 2-3mm, which is the film-forming matrix;
[0026] B. Preparation of the first layer of slow-release film: Mix crisborole 20%, glycerin 25%, microcrystalline cellulose 25%, propylene glycol 7%, carrageenan 8%, and polyvinylpyrrolidone 15%, mix well, melt, melt The material in the state is evenly spread on the above-mentioned prepared film matrix;
[0027] C, the preparation of the second layer of delayed-release film: 20% of crisborole, 25% of propylene alcohol, 25% of sorbitol, 7% of polysorbate, 8% of guar gum, and 15% of polycarbophil are fully mixed, Add an appropriate amount of water to dissolve, stir evenly, spread it evenly on the above slow-...
Embodiment 2
[0030] A. Membrane matrix preparation: Spandex and silk are used as the matrix material to weave the matrix, and the moisture-discharging strips are arranged at intervals on the matrix. The moisture-relieving strips are made of antibacterial yarn and acrylic fiber, and the interval width is 2-3mm. The width of the moisture strip is 2-3mm, which is the film-forming matrix;
[0031] B. Preparation of the first layer of slow-release film: mix crisborole 25%, polyethylene glycol 23%, modified starch 22%, menthol 8%, carboxymethylcellulose sodium 7%, chitosan 15% fully, melt, and evenly smear the material in the molten state on the above-mentioned prepared film matrix;
[0032] C. Preparation of the second delayed-release film: mix crisborole 25%, triacetin 23%, dextrin 22%, menthol 8%, carboxymethylcellulose sodium 7%, and polyvinyl alcohol 15%. Fully, add appropriate amount of water to dissolve, stir evenly, spread it evenly on the above slow-release film, and dry it in a dryer ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com