Preparation method of crisaborole
A technology of criborole and nitrophenylboronic acid, which is applied in the field of preparation of criborole, can solve the problems of low total yield, low yield, many side reactions, etc., and achieves mild and controllable reaction, cheap and easy raw materials. The effect of obtaining, high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0043] The synthetic route of the preparation method of a kind of crisborole that the present invention proposes is as follows:
[0044] With reference to the above-mentioned route, a kind of preparation method of crisborole proposed by the present invention comprises the following steps:
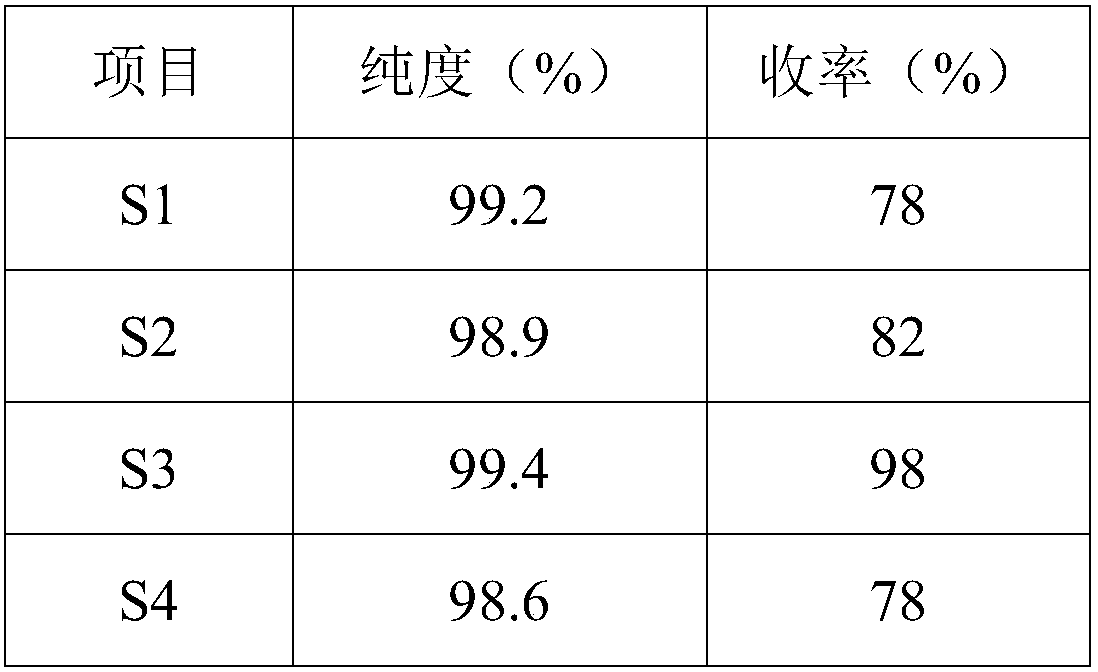
[0045] S1, carrying out nitration reaction with o-cyanophenylboronic acid (2) and concentrated nitric acid to obtain 2-cyano-4-nitrophenylboronic acid (3);
[0046] S2. Reducing 2-cyano-4-nitrophenylboronic acid to obtain 2-formyl-4-nitrophenylboronic acid (4);
[0047] S3, reducing 2-formyl-4-nitrophenylboronic acid to obtain 2-hydroxymethyl-4-nitrophenylboronic acid (5);
[0048] S4, 2-hydroxymethyl-4-nitrophenylboronic acid obtains 2-hydroxymethyl-5-nitrophenylboronic acid half ester (6) through condensation reaction;
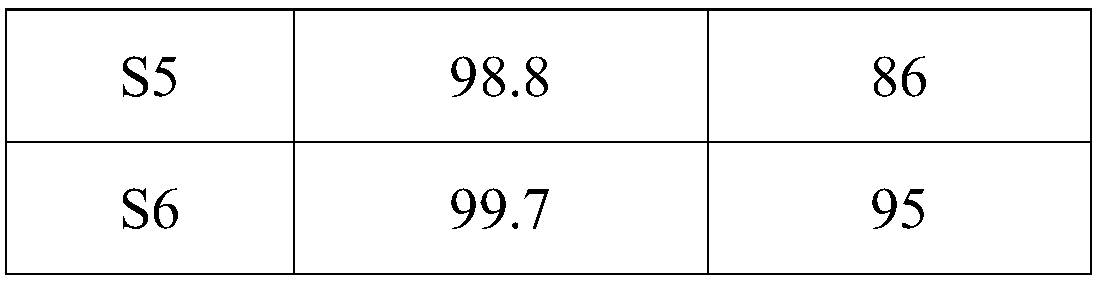
[0049] S5, 2-hydroxymethyl-5-nitrophenylboronic acid half ester undergoes reduction reaction, diazotization hydrolysis reaction to obtain 2-hydroxymethyl-5-hydroxypheny...
Embodiment 1
[0053] A preparation method of crisborole, comprising the steps of:
[0054] Synthesis of S1, 2-cyano-4-nitrophenylboronic acid: Add 1.67kg of concentrated sulfuric acid to a round-bottomed flask equipped with mechanical stirring, slowly add 1.07kg of fuming nitric acid dropwise under an ice-salt bath, and control the temperature of the reaction system Do not exceed 20°C. After the addition, add 500g of 2-cyanophenylboronic acid in batches. After the addition, raise the temperature to 80°C for 5 hours. TLC monitors the progress of the reaction. After the conversion of the raw materials is complete, cool to room temperature and pour the obtained reaction solution into In 5L of ice water, a large number of light yellow solids precipitated, filtered, dried, and recrystallized from absolute ethanol to obtain light yellow 2-cyano-4-nitrophenylboronic acid;
[0055] Synthesis of S2, 2-formyl-4-nitrophenylboronic acid: 0.5kg of 2-cyano-4-nitrophenylboronic acid was added in a round-b...
Embodiment 2
[0061] A preparation method of crisborole, comprising the steps of:
[0062] Synthesis of S1, 2-cyano-4-nitrophenylboronic acid: Add 1.336 kg of concentrated sulfuric acid to a round bottom flask equipped with mechanical stirring, slowly add 0.856 kg of fuming nitric acid dropwise in an ice-salt bath, and control the temperature of the reaction system Do not exceed 20°C. After the addition, add 500g of 2-cyanophenylboronic acid in batches. After the addition, raise the temperature to 40°C for 5 hours. TLC monitors the progress of the reaction. After the conversion of the raw materials is complete, cool to room temperature, and pour the obtained reaction solution In 5L of ice water, a large number of light yellow solids precipitated, filtered, dried, and recrystallized from absolute ethanol to obtain light yellow 2-cyano-4-nitrophenylboronic acid;
[0063] Synthesis of S2, 2-formyl-4-nitrophenylboronic acid: 0.5kg of 2-cyano-4-nitrophenylboronic acid was added in a round-bottom...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com