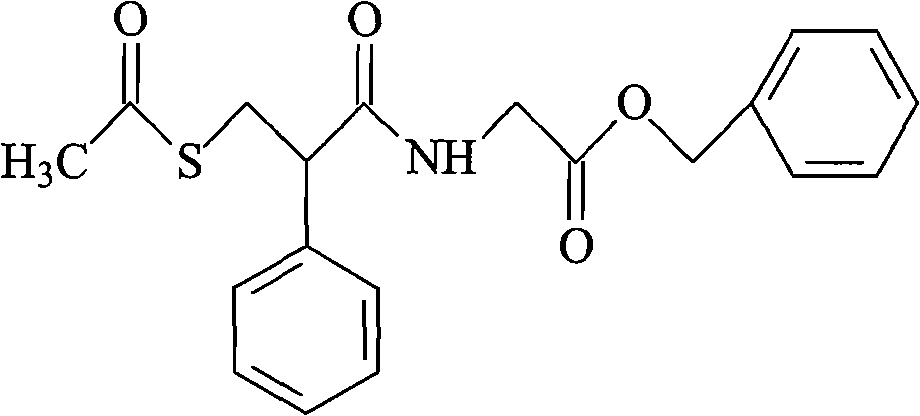
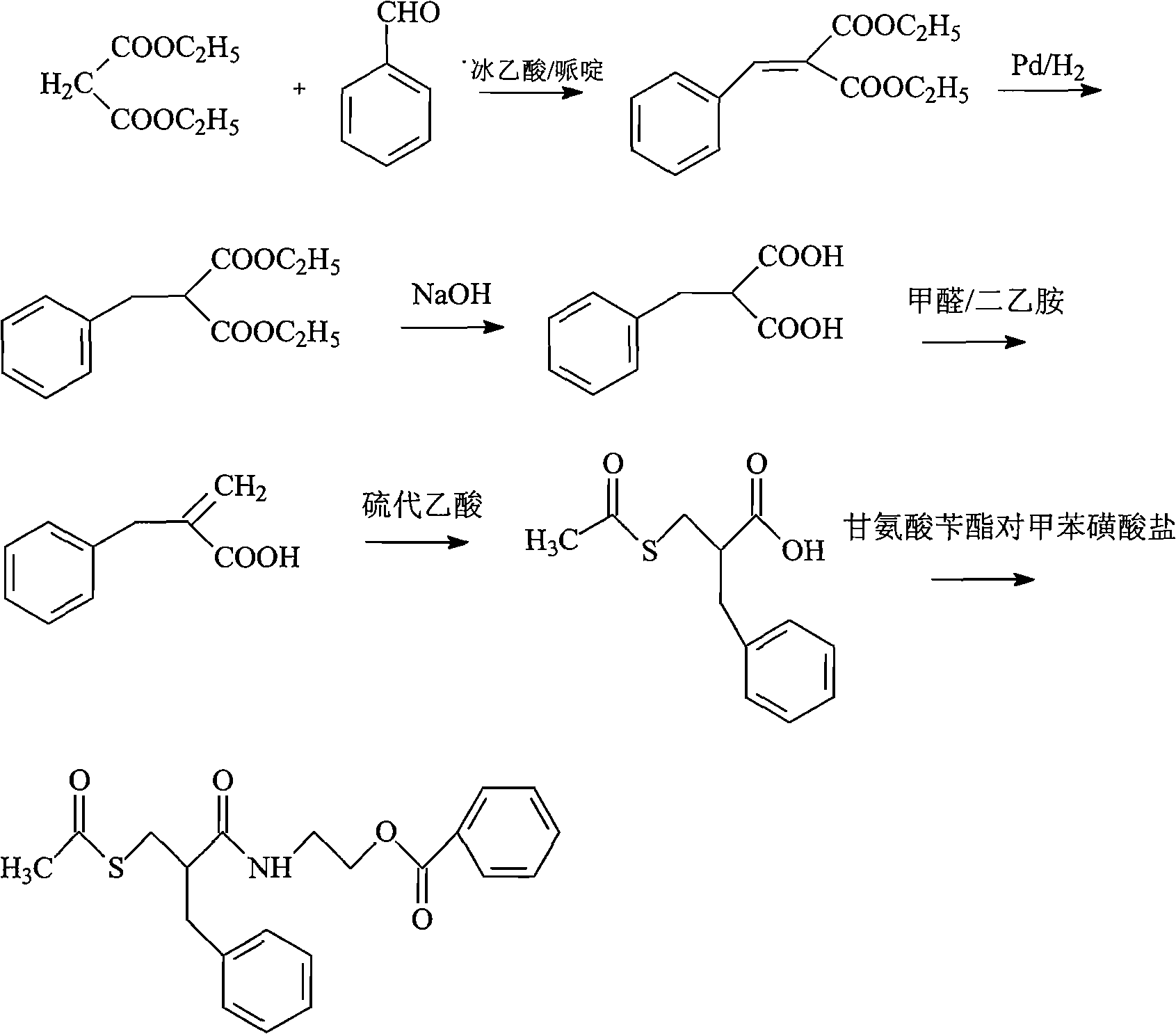
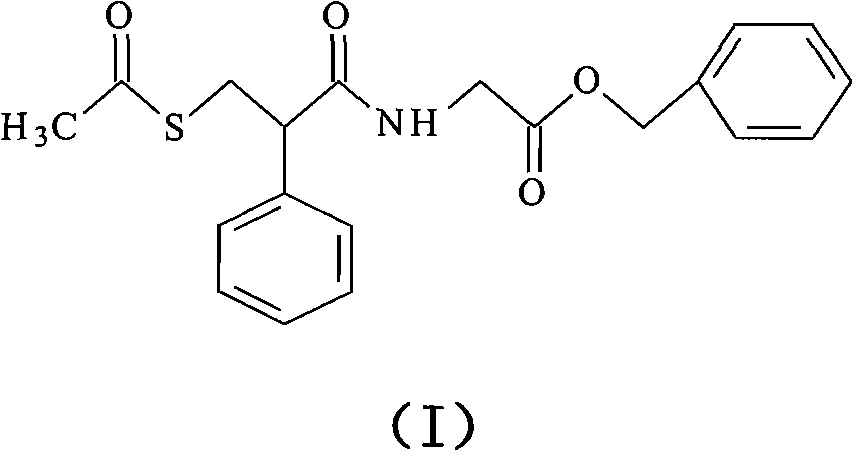
Racecadotril compound and novel preparation method thereof
A technology of racecadotril and compounds, which is applied in the field of medicine, can solve the problems of enhanced color, reduced content of active ingredients in medicines, and low yield of racecadotril, and achieves improved product quality, mild reaction conditions, and reduced toxicity. side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Dissolve 5 g of racecadotril (purity: 97.3%) in 100 ml of ethanol-ethyl acetate (1:5) and add it to the top of the column. The filler is ICN allumina N with a particle size of 18-32 μm and a pore size of 6 nm. permanent alumina. The length of the column is 25cm, the diameter is 5cm, and the column pressure is 0.5pa. Then, ethanol:ethyl acetate (1:5) is pumped into the column for column chromatography. , collected in sections, collected the liquid, and dried in vacuum to obtain 4.7 g of refined racecadotril, with a yield of 96.7% and a purity of 99.7%.
Embodiment 2
[0040] Dissolve 10 g of racecadotril crude product (purity: 96.2%) in 200 ml of ethanol-ethyl acetate (1:9) and add to the top of the column. The filler is ICN allumina N with a particle size of 18-32 μm and a pore size of 6 nm. Fine-pored neutral alumina. The length of the column is 25cm, the diameter is 5cm, the column pressure is 5.0pa, and then pumped into ethanol-ethyl acetate (1:9) for column chromatography, the flow rate is 2.0ml / min, the column temperature is kept at 45°C, start timing, sampling, and follow-up monitoring , collected in sections, collected the liquid, and dried in vacuum to obtain 9.2 g of refined racecadotril, with a yield of 95.6% and a purity of 99.8%.
Embodiment 3
[0042] Dissolve 15 g of racecadotril crude product (purity: 98.0%) in 200 ml of ethanol-ethyl acetate (1:1) and add to the top of the column. The filler is Baker pores with a particle size of 50-200 μm and a pore size of 6 nm permanent alumina. The length of the column is 25cm, the diameter is 5cm, the column pressure is 1.5pa, and then pumped into ethanol-ethyl acetate (1:1) for column chromatography, the flow rate is 1.5ml / min, the column temperature is kept at 40°C, start timing, sampling, and follow-up monitoring , collected in sections, collected the liquid, and dried in vacuum to obtain 14.0 g of refined racecadotril, with a yield of 95.2% and a purity of 99.7%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com