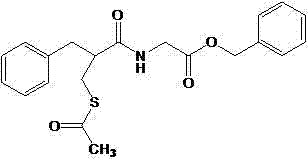
Racecadotril particle as well as preparation method thereof
A technology of racecadotril and granules, which is applied to the digestive system, pharmaceutical formulations, medical preparations of inactive ingredients, etc., can solve the problems of bad taste of racecadotril, difficulty in taking medicine for children, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The racecadotril cyclodextrin inclusion compound of the present invention is composed of the following materials in weight ratio, racecadotril:β-cyclodextrin=1:5.
[0027] (1) Weigh 50g of β-cyclodextrin according to the above weight ratio, dissolve it in 250g of water, and grind it into a paste, remove 10g of rocadotril, dissolve it with an appropriate amount of ether, add it to the above paste, grind it for 2 hours, and wave off the ether , dried in vacuum, that is.
[0028] components Dosage (g) Racecadotril cyclodextrin inclusion complex 10 sucrose 78 Hydroxypropyl Cellulose 2 Low-substituted hydroxypropyl cellulose 10 Anhydrous Colloidal Silica 0.2 Acesulfame K 0.02
[0029] (2) Adhesive preparation: add an appropriate amount of hydroxypropyl cellulose to water, swell completely, and prepare a 5% adhesive for later use.
[0030] (3) Preparation of granules: Mix racecadotril cyclodextrin or cyclodextrin inclusi...
Embodiment 2
[0032] The racecadotril cyclodextrin inclusion compound of the present invention is composed of the following materials in weight ratio, racecadotril:γ-cyclodextrin=1:10.
[0033] components Dosage (g) Racecadotril cyclodextrin inclusion complex 12 lactose 80 hypromellose 2 Croscarmellose Sodium 6 Anhydrous Colloidal Silica 0.20 stevioside 0.03
Embodiment 3
[0035] The racecadotril cyclodextrin inclusion compound of the present invention is composed of the following materials in weight ratio, racecadotril:hydroxypropyl-β-cyclodextrin=1:20.
[0036] components Dosage (g) Racecadotril cyclodextrin inclusion complex 20 lactose 67 Ethyl cellulose 3 Crospovidone 10 Magnesium stearate 0.20 stevioside 0.05
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com