Alpha crystal form of racecadotril and preparation method of alpha crystal form
A technology for racecadotril and crystal form, which is applied in the field of drug crystal form and preparation, can solve problems such as unreported crystal form, and achieve the effects of high crystal form purity, easy implementation and stable crystal form
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Dissolve 40.0g of racecadotril in 200ml of absolute ethanol, heat in a water bath to 40°C, stir until completely dissolved, stop stirring, place the solution in a constant temperature water bath at 15°C, and when crystals begin to precipitate, then Placed at 0°C for crystallization, filtered, and dried with hot air circulation at 45°C for 6 hours to obtain 29.2 g of racecadotril α crystals with a purity of 99.6%.
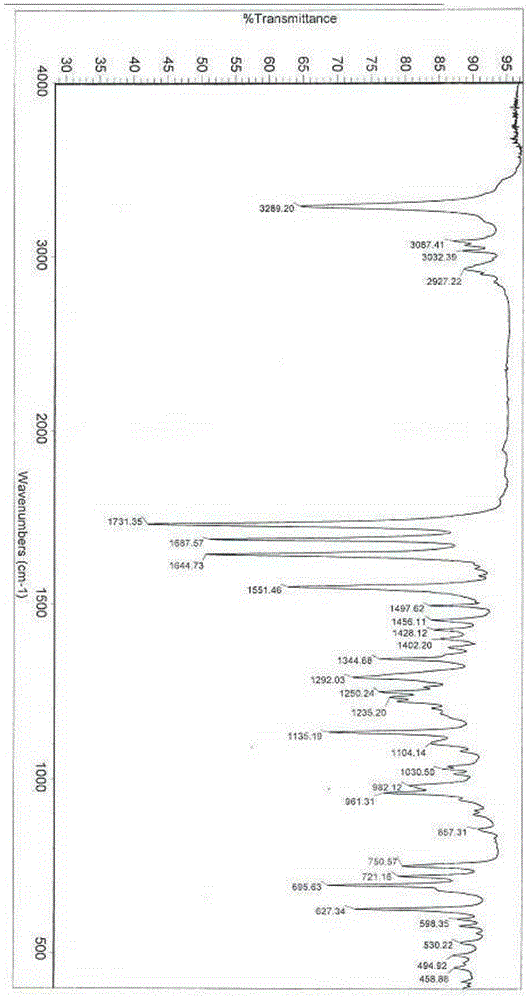
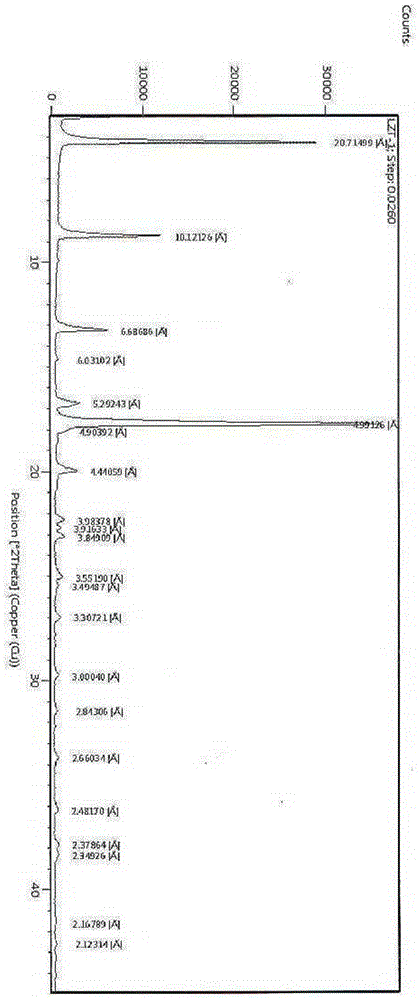
[0033] The reflection angle 2θ of its X-ray powder diffraction pattern shows X-ray powder diffraction peaks at 4.3°, 8.7°, 13.2°, 16.8°, 17.8° and 20.0°. at 1135.19cm -1 、1551.46cm -1 、1644.73cm -1 、1687.57cm -1 、1731.35cm -1 and 3289.20cm -1 shows an infrared absorption peak.
Embodiment 2
[0035] Dissolve 40.0g of racecadotril in 400ml of 85% ethanol aqueous solution, heat in a water bath to 40°C, stir until completely dissolved, stop stirring, and place the solution in a constant temperature water bath at 20°C to stand still, when crystals begin to precipitate , and then placed at 5°C for crystallization, filtered, and dried with hot air circulation at 43°C for 6 hours to obtain 28.5 g of racecadotril α crystals with a purity of 99.4%.
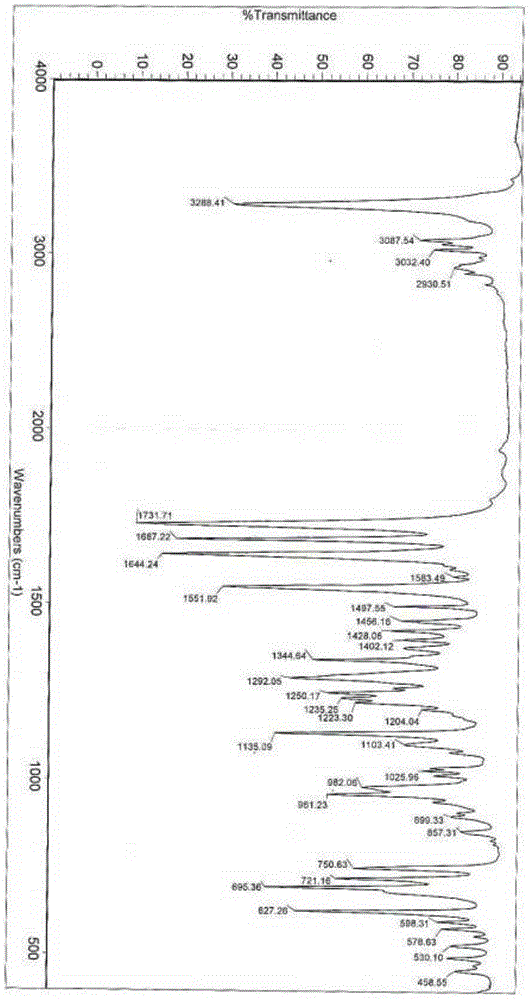
[0036] The reflection angle 2θ of its X-ray powder diffraction pattern shows X-ray powder diffraction peaks at 4.3°, 8.7°, 13.3°, 16.7°, 17.8° and 20.0°. at 1135.09cm -1 、1551.92cm -1 、1644.24cm -1 、1687.22cm -1 、1731.71cm -1 and 3288.41m -1 shows an infrared absorption peak.
Embodiment 3
[0038] Dissolve 40.0g of racecadotril in 400ml of 70% ethanol aqueous solution, heat in a water bath to 45°C, stir until completely dissolved, stop stirring, put the solution in a constant temperature water bath at 25°C and let it stand, when crystals begin to precipitate , and then placed at 5° C. for crystallization, filtered, and dried with hot air circulation at 45° C. for 7 hours to obtain 30.4 g of racecadotril α crystals with a purity of 99.7%.
[0039] The reflection angle 2θ of its X-ray powder diffraction pattern shows X-ray powder diffraction peaks at 4.3°, 8.7°, 13.2°, 16.7°, 17.7° and 20.0°. at 1135.10cm -1 、1552.05cm -1 、1644.21m -1 、1687.28cm -1 、1731.50cm -1 and 3287.80cm -1 shows an infrared absorption peak.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com