Intermediate compound for preparing dextro cadotril and its preparation process and use
A kind of compound, halogenation reaction technology, applied in the field of synthetic drug dexcadotril
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
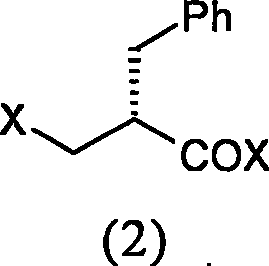
[0041] Preparation of (S)-2-chloromethylphenylpropionyl chloride
[0042] I Halogenated with thionyl halides
[0043] Add 1.4ml (19.2mmol) thionyl chloride and 3 drops of DMF to a 25ml three-necked reaction flask, stir for 5 minutes, cool in an ice bath, add 1.0g (5.6mmol) (S)-2-benzyl-3-hydroxy Propionic acid was reacted at this temperature for 30 minutes, then raised to 60° C. for 1 hour, TLC judged that the reaction was complete, and the solution was evaporated to dryness under reduced pressure to obtain 1.0 g of a colorless oil. MS (EI): 217 (M + )
[0044] II Halogenated with phosphorus halide
[0045] Add 2.0g (11.2mmol) (S)-2-benzyl-3-hydroxypropionic acid into a 50ml three-necked reaction flask equipped with a drying tube, then add 20ml of dichloroethane and stir to dissolve, and cool in an ice bath to -10 After adding 3.5gPCl 5 , maintained the temperature for 30 minutes, then raised the temperature to 60°C for 20 minutes, TLC judged that the reaction was complet...
Embodiment 2
[0049] Preparation of (S)-2-bromomethylphenylpropionyl bromide
[0050] 1. Add 2.0g (11.2mmol) (S)-2-benzyl-3-hydroxypropionic acid into a 25ml three-necked reaction flask, add 10ml of dichloromethane and 1% pyridine, and stir to dissolve. Cool in an ice bath to -5°C, add 5.84 g (28 mmol) of thionyl bromide dropwise, maintain the temperature for 50 minutes, and evaporate the solution to dryness under reduced pressure to obtain 1.8 g of a colorless oil. MS(EI): 306(M + )
[0051] II Add 2.0g (4.5mmol) (S)-2-benzyl-3-hydroxypropionic acid to a 25ml three-necked reaction flask, add 10ml dichloroethane, and stir to dissolve. After cooling to -10°C in an ice bath, 4.5 g of boron tribromide was added, and the reaction was maintained at this temperature for 30 minutes, and the temperature was raised to 60°C for 20 minutes. TLC judged that the reaction was complete, and the solution was evaporated to dryness under reduced pressure to obtain 2.2 g of a colorless oil.
[0052] III Ad...
Embodiment 3
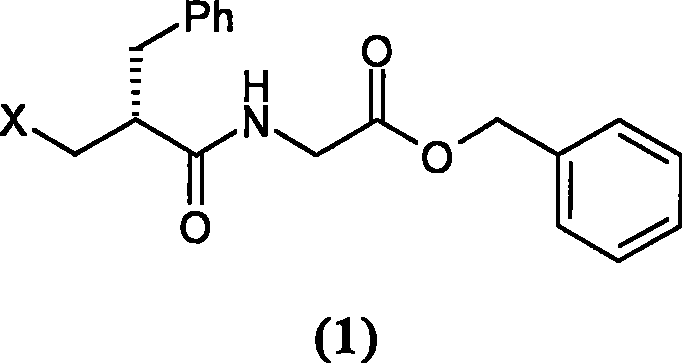
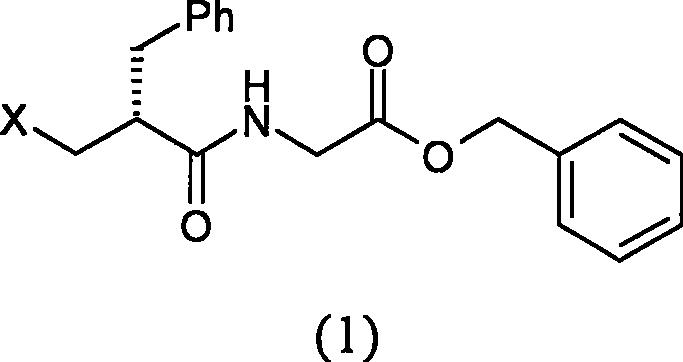
[0054] Preparation of N-[(2R)-2-chloromethyl-1-oxo-3-phenylpropyl]glycine benzyl ester
[0055] Add 1.9g (5.6mmol) p-toluenesulfonate of glycine benzyl ester and 60ml dichloromethane into a 100ml three-necked flask, cool in an ice bath to 0°C, add 4ml triethylamine, stir for 10 minutes, add dropwise 30ml dissolved in 1.1 g (5.1 mmol) of (S)-2-chloromethylphenylpropionyl chloride in dichloromethane. Reacted for 2 hours, TLC judged that the reaction was complete, washed twice with water, dried over anhydrous sodium sulfate, filtered, concentrated to dryness, stirred and crystallized with 30ml of petroleum ether to obtain 1.5g of solid (yield 86.1%). mp88℃
[0056] 1 HNMR (CDCl 3 ): 7.16-7.39 (10H, m), 5.91 (1H, br), 5.17 (2H, m), 3.94-4.12 (2H, m), 3.56-3.78 (2H, m), 2.88-3.02 (2H, m ), 2.74-2.81 (1H, m)
[0057] MS (EI): 345 (M + )
[0058] [α] D 23 (c1.05, methanol) = -12.0°
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com