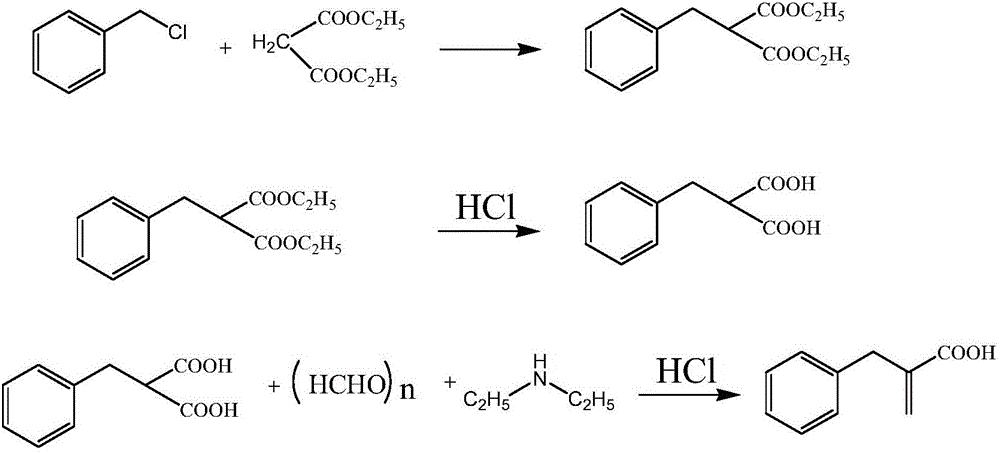
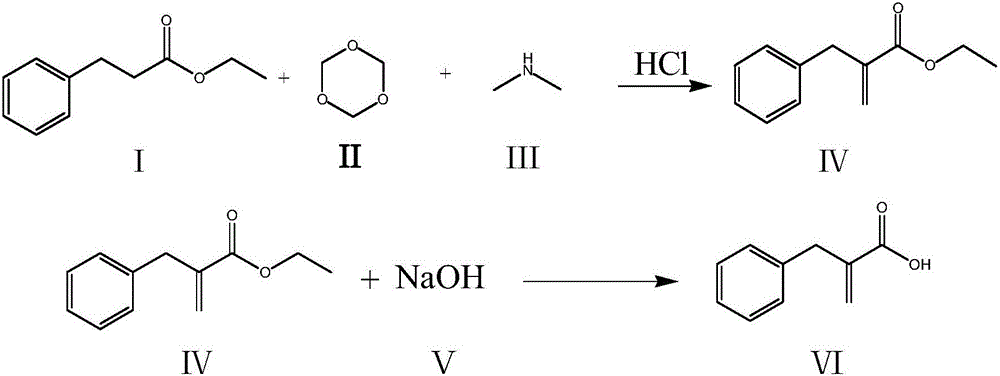
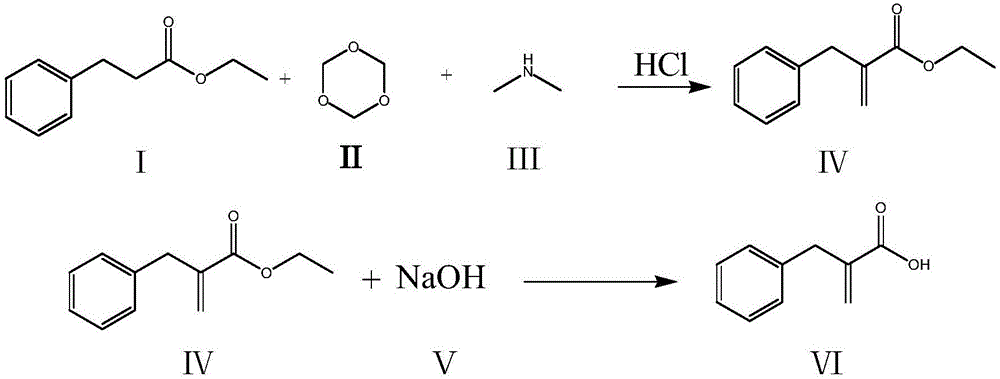
Compounding method for racecadotril intermediate
A racecadotril and synthesis method technology, applied in the field of drug synthesis, can solve the problems of unfavorable industrial production, production environmental pollution, long synthetic route, etc., and achieve the effects of easy industrial production, high purity and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] (1) Add 70g of ethyl phenylpropionate and 400ml of ethyl acetate to a 1000ml reaction bottle, stir evenly, cool down, control the temperature in the reaction bottle at 0-10°C, add 54.7g of dimethylamine with a concentration of 40% dropwise, and the addition is completed , and react at a temperature of 10-15°C for 1 hour, then add 14.2g of paraformaldehyde, heat up and then reflux for 3 hours; after the reaction is completed, cool down to room temperature, add 56g of concentrated hydrochloric acid (concentration 35%, the same below) and 60ml of purified water, Stir for 40 minutes, stand to separate layers, wash the organic layer once with 200ml purified water, and concentrate the organic layer to dryness under reduced pressure;
[0025] (2) Then add 500ml of dichloromethane, 80g of 20% sodium hydroxide solution and 50g of absolute ethanol, and react at a temperature of 10-20°C for 4 hours; pH=1; separate the water layer again, wash the organic layer with 300ml of purifie...
Embodiment 2
[0027] (1) Add 70g of ethyl phenylpropionate and 400ml of ethyl acetate to a 1000ml reaction bottle, stir evenly, cool down, control the temperature in the reaction bottle at 0-10°C, add 55.0g of dimethylamine with a concentration of 40% dropwise, and the addition is completed , temperature control 10-15 ℃ for 50 minutes, then add 14.5g of paraformaldehyde, heat up and then reflux for 2.5 hours; after the reaction is completed, cool down to room temperature, add 58g of concentrated hydrochloric acid (concentration 35%), 65ml of purified water, and stir for 40 minutes , standing to separate layers, the organic layer was washed once with 200ml purified water, and the organic layer was concentrated under reduced pressure to dryness;
[0028] (2) Then add 500ml of methylene chloride, 85g of 20% sodium hydroxide solution and 50g of absolute ethanol, and react at a temperature of 10-20°C for 3.5 hours; after standing, separate the water layer, and add 45g of concentrated hydrochloric...
Embodiment 3
[0030] (1) Add 70g of ethyl phenylpropionate and 400ml of ethyl acetate to a 1000ml reaction bottle, stir evenly, cool down, control the temperature in the reaction bottle at 0-10°C, add 54.5g of dimethylamine with a concentration of 40% dropwise, and the addition is completed , and react at a temperature of 10-15°C for 70 minutes, then add 14.0g of paraformaldehyde, heat up and then reflux for 3.5 hours; after the reaction is completed, cool down to room temperature, add 55g of concentrated hydrochloric acid (concentration 35%), 60ml of purified water, and stir for 40 minutes , standing to separate layers, the organic layer was washed once with 200ml purified water, and the organic layer was concentrated under reduced pressure to dryness;
[0031] (2) Then add 500ml of dichloromethane, 80g of 20% sodium hydroxide solution and 50g of absolute ethanol, and react at a temperature of 10-20°C for 4 hours; pH=1; separate the water layer again, wash the organic layer with 300ml of p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com