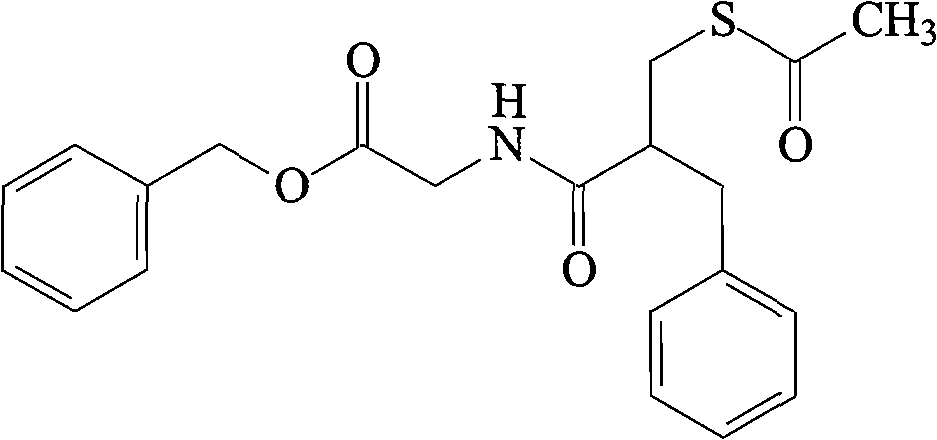
Racecadotril liposome solid preparation
A technology for racecadotril and solid preparation, applied in the field of medicine, can solve the problems of affecting the speed and effect of drug treatment, not improving the solubility of active ingredients, low solubility of racecadotril, etc., so as to improve the bioavailability and reduce the Toxic and side effects, the effect of improving solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1 Preparation of racemic cardotril liposome tablets
[0043] Prescription (1000 tablets)
[0044]
[0045]
[0046] Preparation Process
[0047] (1) Dissolve 10g racecadotril, 25g soybean lecithin, 25g hydrogenated egg yolk lecithin, 18g cholesterol and 6g Tween 80 in 800ml mixed solvent of ethanol and isopropanol with a volume ratio of 2:1 to obtain Lipid solution
[0048] (2) Put the above-mentioned lipid solution in a pear-shaped bottle, and spin-evaporate in a constant temperature water bath at 55°C to remove the mixed solvent to form a uniform lipid film;
[0049] (3) Prepare 500ml of phosphate buffer solution with pH 6.0, add it to a pear-shaped bottle and shake it gently to elute the lipid membrane and disperse it in the hydration medium to dissolve, to obtain a liposome suspension;
[0050] (4) Pour the above suspension into the APV2000 milk homogenizer, and continue milk homogenization under a pressure of 0.5Mpa;
[0051] (5) Filter the above suspension with a 0.4...
Embodiment 2
[0055] Example 2 Preparation of racemic cardotril liposome particles
[0056] Prescription (1000 bags)
[0057]
[0058]
[0059] Preparation Process
[0060] (1) Dissolve 30g of racecadotril, 120g of soybean lecithin, 120g of hydrogenated egg yolk lecithin, 100g of cholesterol and 45g of Tween 80 in 2000ml of a mixed solvent of ethanol and isopropanol with a volume ratio of 2:1 to obtain Lipid solution
[0061] (2) Put the above-mentioned lipid solution in a pear-shaped bottle, and spin-evaporate in a constant temperature water bath at 45°C to remove the mixed solvent to form a uniform lipid film;
[0062] (3) Prepare 1000ml of phosphate buffer solution with pH 6.0, add it to a pear-shaped bottle and shake it gently to elute the lipid membrane and disperse it in the hydration medium to dissolve, to obtain a liposome suspension;
[0063] (4) Pour the above suspension into the APV2000 milk homogenizer, and continue milk homogenization under a pressure of 2.8Mpa;
[0064] (5) Filter the ab...
Embodiment 3
[0066] (7) Bagging to prepare racecadotril liposome granules. Example 3 Preparation of racemic cardotril liposome capsules
[0067] Prescription (1000 capsules)
[0068]
[0069]
[0070] Preparation Process
[0071] (1) Dissolve 100 g of racecadotril, 600 g of soybean lecithin, 600 g of hydrogenated egg yolk lecithin, 500 g of cholesterol and 200 g of Tween 80 in 10000 ml of a mixed solvent of ethanol and isopropanol with a volume ratio of 2:1 to obtain Lipid solution
[0072] (2) Put the above-mentioned lipid solution in a pear-shaped bottle and spin-evaporate in a constant temperature water bath at 50°C to remove the mixed solvent to form a uniform lipid film;
[0073] (3) Prepare 5000ml of phosphate buffer solution with pH 6.0, add it to a pear-shaped bottle and shake it gently to elute the lipid membrane and disperse it in the hydration medium to dissolve, to obtain a liposome suspension;
[0074] (4) Pour the above suspension into the APV2000 milk homogenizer, and continuously ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com