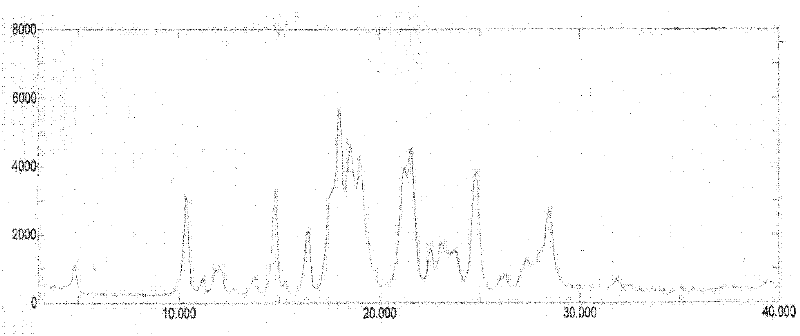
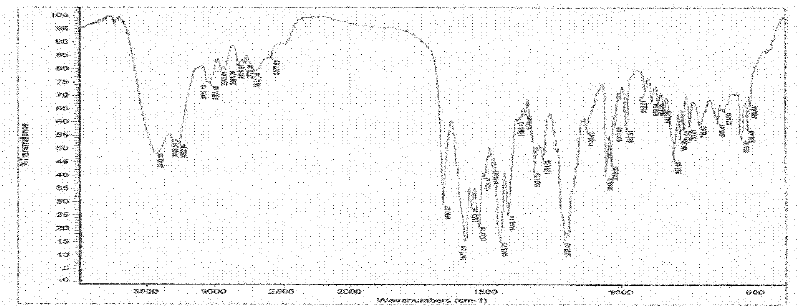
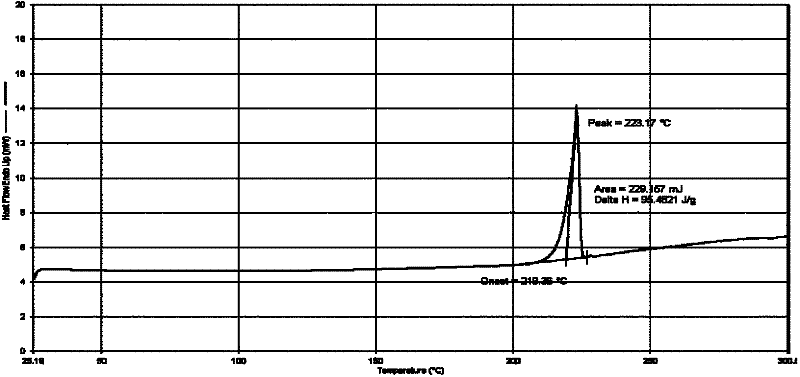
Imatinib mesylate polymorph and pharmaceutical composition thereof
A technology of imatinib mesylate and polymorphic form, which is used in drug combinations, antipyretics, anti-infectives, etc., can solve the problem of inability to ensure effective removal of mechanical impurities, unstable reproducibility, and poor product purity and other problems, to achieve the effect of suitable for industrial scale preparation, good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] The preparation of embodiment 1 imatinib mesylate crude product
[0116] Add imatinib (2Kg), methanesulfonic acid (390g) and acetone (20L) into the reaction flask, heat up to reflux for 2.5 hours under stirring. Cool down to room temperature, stir and crystallize for 3 hours. After suction filtration, the obtained filter cake was collected and dried under reduced pressure at 70°C to constant weight to obtain 2.2Kg of crude imatinib mesylate. Yield, 92.1%.
[0117] Purity: 98.7%.
Embodiment 2
[0118] The preparation of embodiment 2 polymorph II
[0119] Preparation of imatinib free base
[0120] The crude imatinib mesylate obtained in Example 1 (1.5 Kg) was added into water (15 L), heated to 60° C., and stirred to dissolve. Suction to remove impurities. The filtrate was cooled to room temperature, and acetone (4.5 L) was added with stirring. Sodium bicarbonate (214 g) was added and crystallized at room temperature for 3 hours. After suction filtration, the filter cake was washed with water and dried to constant weight to obtain 1.17Kg of imatinib. Yield: 92.9%.
[0121] Preparation of imatinib mesylate
[0122] The imatinib (1Kg) obtained above, methanesulfonic acid (195g) and methyl isobutyl ketone (10L) were added into the reaction flask, and the temperature was raised to reflux for 2 hours under stirring. Cool down to room temperature, stir and crystallize for 1 hour. After suction filtration, the obtained filter cake was collected and dried under reduced ...
Embodiment 3
[0126] The preparation of embodiment 3 polymorph II
[0127] According to the existing method, we used imatinib mesylate of different crystal forms prepared by different processes as raw materials, and adopted the scheme of Example 2 to prepare the data list of polymorph II as follows:
[0128]
[0129]
[0130] It can be seen from the above test data that the crude product of imatinib mesylate in any crystal form can be converted into the crystal form of the present invention. At the same time, impurities can be effectively removed to obtain high-purity imatinib mesylate up to 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com