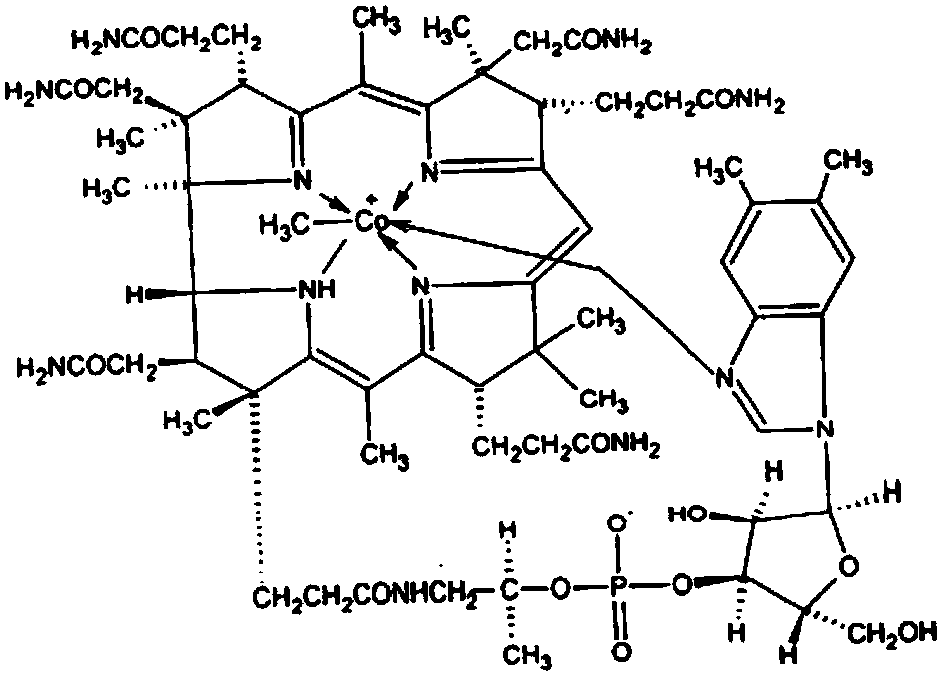
Method used for preparing mecobalamin
A technology for methylcobalamin and cyanocobalamin, applied in the field of preparing methylcobalamin, can solve the problems of easy generation of other cobalamin, weak methylation ability, unsuitable for production, etc., and achieves significant economic value and methylation ability. Strong, less toxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] A method for preparing methylcobalamin, comprising the following steps:
[0050] 1. Reduction reaction: Add 8.0g of cyanocobalamin, 0.3g of cobalt chloride hexahydrate, 6ml of methyl ethyl ketone, and 104ml of water into the flask under the condition of avoiding light, and protect with nitrogen, control the temperature at 30-40°C, stir and dissolve, An aqueous solution of sodium borohydride (3.2 g of sodium borohydride dissolved in 16 ml of water) was slowly added dropwise to the reaction solution at a rate of 4 ml / min. After the addition was complete, the stirring was continued for 30 minutes;
[0051] 2. Methylation reaction: adjust the temperature of the reaction solution to 35-45°C, and immediately add the prepared trimethylsulfoxide iodide solution (2.6g trimethylsulfoxide iodide dissolved in 24ml water) dropwise into the reaction solution , continue stirring for 20 minutes after the dropwise addition;
[0052] 3. Crystallization: Use a mixture of acetone and acet...
Embodiment 2
[0056] A method for preparing methylcobalamin, comprising the following steps:
[0057] 1. Reduction reaction: Add 40.0g of cyanocobalamin, 2.4g of cobalt chloride hexahydrate, 30ml of methyl ethyl ketone, and 500ml of water into the flask under the condition of avoiding light, protect with nitrogen, control the temperature at 30-40°C, and stir to dissolve. An aqueous solution of sodium borohydride (30 g of sodium borohydride dissolved in 300 ml of water) was slowly added dropwise into the reaction solution at a rate of 8 ml / min. After the addition was complete, the stirring was continued for 30 minutes;
[0058] 2. Methylation reaction: adjust the temperature of the reaction solution to 35-45°C, immediately add the prepared trimethylsulfoxide iodide solution (16g trimethylsulfoxide iodide dissolved in 160ml water) dropwise into the reaction solution, Continue stirring for 20 minutes after the dropwise addition;
[0059] 3. Crystallization: Use a mixture of acetone and acetic...
Embodiment 3
[0063] A method for preparing methylcobalamin, comprising the following steps:
[0064] 1. Reduction reaction: Add 20g of cyanocobalamin, 1.0g of cobalt chloride hexahydrate, 16ml of methyl ethyl ketone, and 200ml of water into the flask under the condition of avoiding light. Sodium borohydride aqueous solution (12g of sodium borohydride dissolved in 60ml of water) was slowly added dropwise to the reaction solution at a rate of 6ml / min. After the addition was complete, the stirring was continued for 30 minutes;
[0065] 2. Methylation reaction: adjust the temperature of the reaction solution to 35-45°C, immediately add the prepared trimethylsulfoxide iodide solution (10g trimethylsulfoxide iodide dissolved in 100ml water) dropwise into the reaction solution, Continue stirring for 20 minutes after the dropwise addition;
[0066] 3. Crystallization: Use a mixture of acetone and acetic acid (the volume ratio of acetone and acetic acid is 1:1) to adjust the pH of the reaction sol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com