Dopamine prodrug composition and preparation method thereof
A technology of composition and dopamine, which is applied in the field of medicine, can solve the problems that the quality of finished products cannot meet the quality standards, the components of the composition and the preparation method are complicated, and the stability problems such as product discoloration are covered up, and the amount of excipients is cheap, easy to obtain, and stable. Excellent performance and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
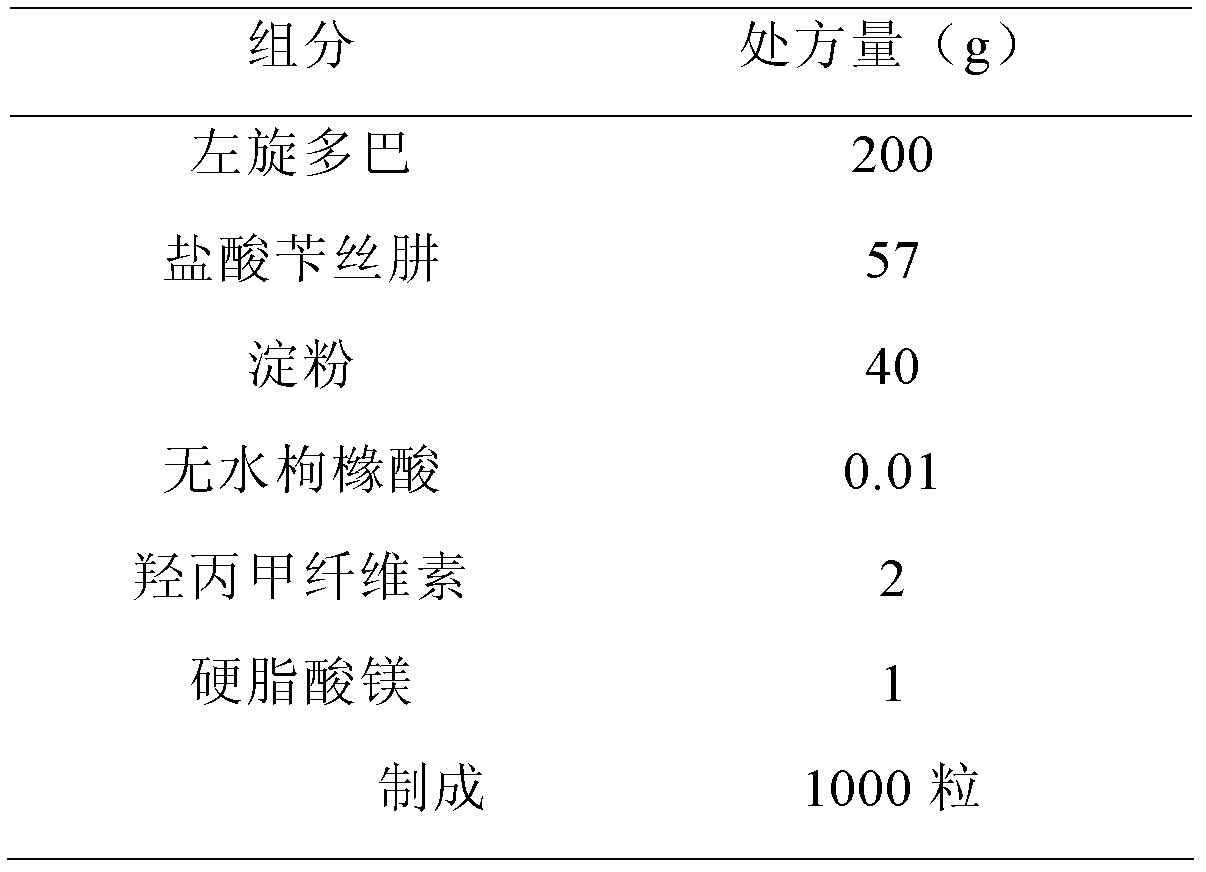
[0083]
[0084] Preparation:
[0085] The hypromellose was configured as a 5% aqueous solution, and anhydrous citric acid was added and stirred until dissolved. Put levodopa, benserazide hydrochloride, and starch into a PMA-25 high-speed stirring granulator (GEA Company, Germany) after crushing and sieving, start mixing, add slurry soft materials, and pass through a YK-60 oscillating granulator (Shanghai) Tianxiang Jiantai Pharmaceutical Machinery Co., Ltd.) sieves the wet granules, then the wet granules are dried on the FL-5 type fluidized granulator (Shanghai Huafa Pharmaceutical Machinery Complete Set Development Company), granulated, and magnesium stearate is added. After mixing by a V-type high-efficiency mixer (Shanghai Tianhe Pharmaceutical Machinery Co., Ltd.), GKF-120 capsule filling machine (Bosch Packaging Technology (Hangzhou) Co., Ltd.) fills the capsules.
Embodiment 2
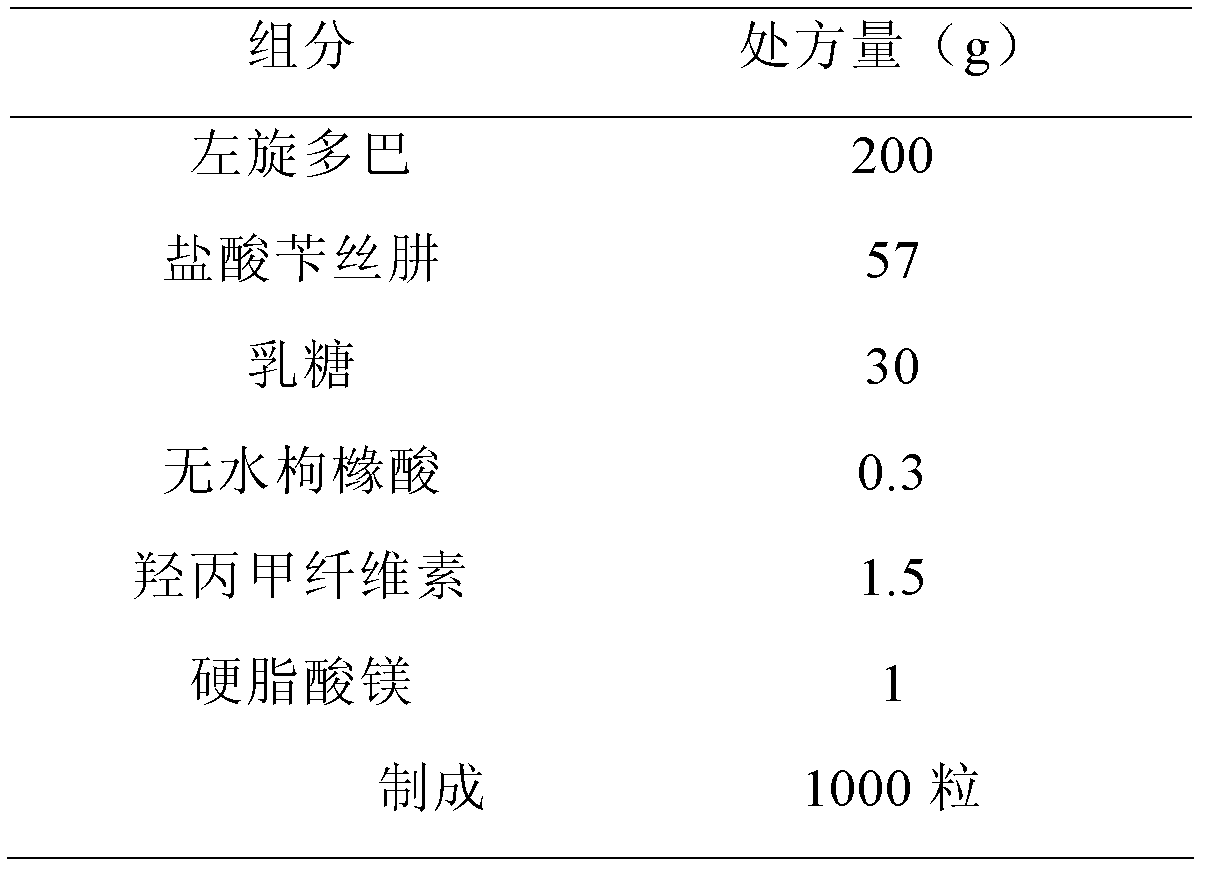
[0087]
[0088] Preparation:
[0089] The hypromellose was configured as a 4% aqueous solution, and anhydrous citric acid was added and stirred until dissolved. Put levodopa, benserazide hydrochloride, and starch into a PMA-25 high-speed stirring granulator (GEA Company, Germany) after crushing and sieving, start mixing, add slurry soft materials, and pass through a YK-60 oscillating granulator (Shanghai) Tianxiang Jiantai Pharmaceutical Machinery Co., Ltd.) sieves the wet granules, then the wet granules are dried on the FL-5 type fluidized granulator (Shanghai Huafa Pharmaceutical Machinery Complete Set Development Company), granulated, and magnesium stearate is added. After mixing by a V-type high-efficiency mixer (Shanghai Tianhe Pharmaceutical Machinery Co., Ltd.), GKF-120 capsule filling machine (Bosch Packaging Technology (Hangzhou) Co., Ltd.) fills the capsules.
Embodiment 3
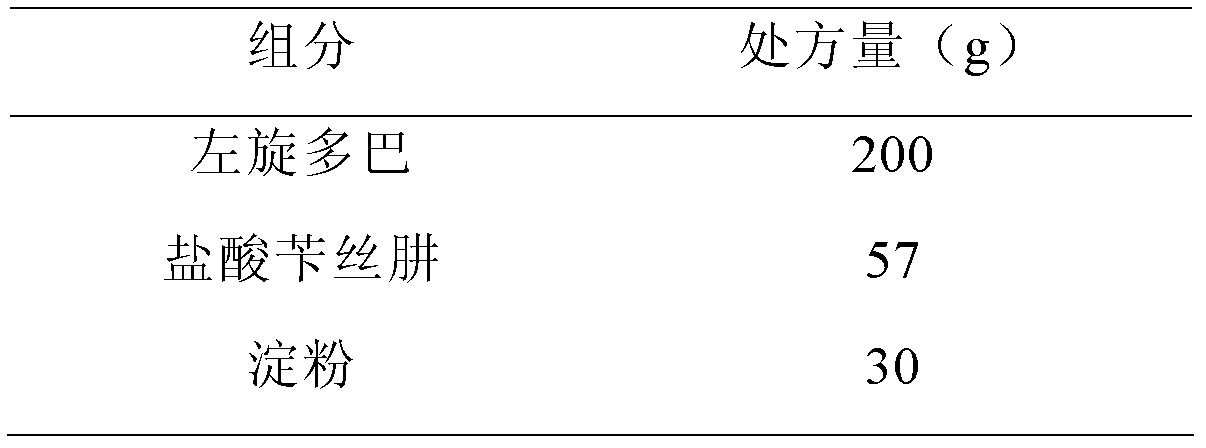
[0091]
[0092]
[0093] Preparation:
[0094] The hypromellose was configured as a 3% aqueous solution, and anhydrous citric acid was added and stirred until dissolved. Put levodopa, benserazide hydrochloride, and starch into a PMA-25 high-speed stirring granulator (GEA Company, Germany) after crushing and sieving, start mixing, add slurry soft materials, and pass through a YK-60 oscillating granulator (Shanghai) Tianxiang Jiantai Pharmaceutical Machinery Co., Ltd.) sieves wet granules, and then dries the wet granules in a DHG-9246A electric heating constant temperature blast drying oven (Shanghai Jinghong Experimental Equipment Co., Ltd.), granulates, and adds stearic acid Magnesium, after mixing by a V-type high-efficiency mixer (Shanghai Tianhe Pharmaceutical Machinery Co., Ltd.), GKF-120 capsule filling machine (Bosch Packaging Technology (Hangzhou) Co., Ltd.) to fill the capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com