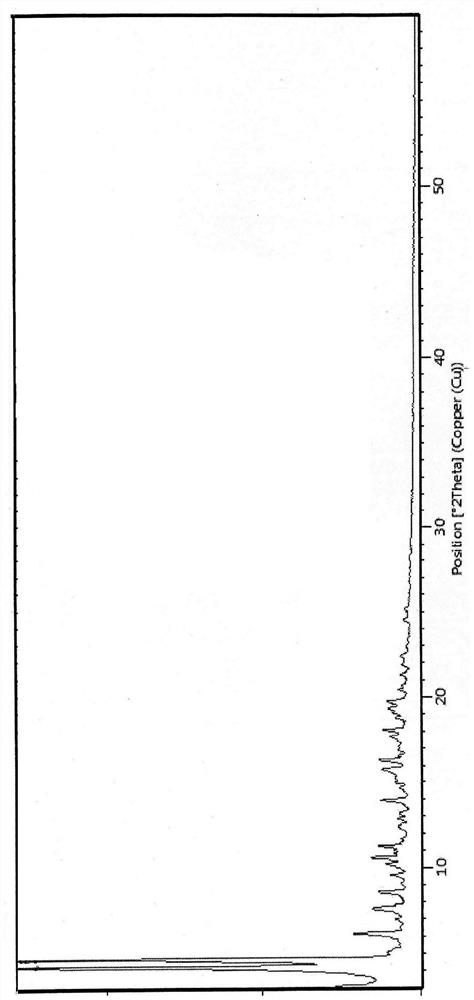
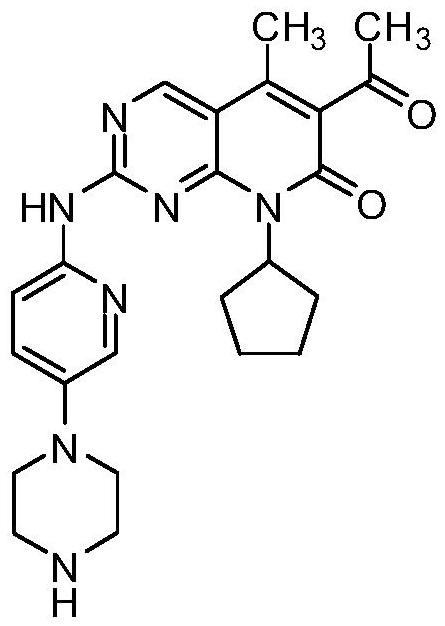
Novel intermediate of palbociclib, and crystal form and preparation method of novel intermediate
A crystal form, piperazine-based technology, applied in the field of medicinal chemistry, can solve the problems of high process cost, low yield, unsuitable for industrial production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
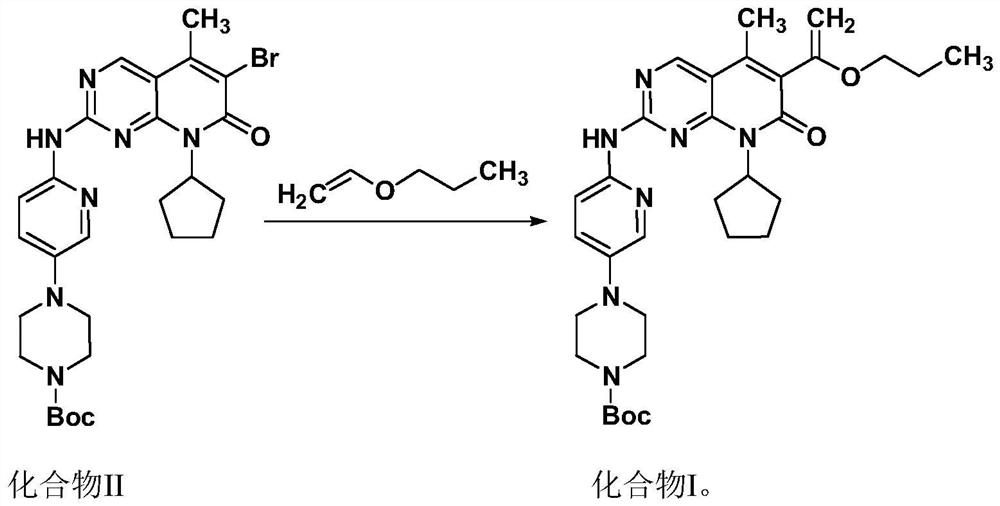
[0037] Example 1, 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-(1-propoxyvinyl)-7,8-dihydropyridine Preparation of [2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)-piperazinyl-1-carboxylic acid tert-butyl ester
[0038] Under nitrogen protection, 180ml of n-propanol was added into the reaction flask, and the stirring was started. 20.0 g of compound II and 10.6 g of diisopropylethylamine were added. 9.0 g of propyl vinyl ether, 0.3 g of palladium acetate and 0.9 g of bis(2-phenylphosphorylphenyl)ether were added. The temperature was raised to 85-95° C., and after 5 hours of incubation, the reaction was detected by TLC. Add 180 ml of n-heptane. Cool down to 5-15°C, keep warm and crystallize for 2.5h. Suction filter and wash with n-propanol-n-heptane mixed solution. After drying at 75°C for 5 h, 18.4 g of compound I was obtained, with a yield of 91.2% and a purity of 99.5%.
Embodiment 2
[0039] Example 2, 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-(1-propoxyvinyl)-7,8-dihydropyridine Preparation of [2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)-piperazinyl-1-carboxylic acid tert-butyl ester
[0040] Under nitrogen protection, 160ml of ethanol was added into the reaction flask, and the stirring was started. 20.0 g of compound II and 10.6 g of diisopropylethylamine were added. 9.0 g of propyl vinyl ether, 0.3 g of palladium acetate and 0.9 g of bis(2-phenylphosphorylphenyl)ether were added. The temperature was raised to 75-78°C, and after 8 hours of incubation, the reaction was detected by TLC. 180 ml of isopropyl ether was added. Cool down to 5-15°C, keep warm and crystallize for 2.5h; filter with suction and wash with a mixed solution of isopropyl ether. After drying at 75°C for 5 h, 18.8 g of compound I was obtained, with a yield of 93.2% and a purity of 99.2%.
Embodiment 3
[0041] Example 3, 4-(6-(8-cyclopentyl-5-methyl-7-oxo-6-(1-propoxyvinyl)-7,8-dihydropyridine Preparation of [2,3-d]pyrimidin-2-ylamino)pyridin-3-yl)-piperazinyl-1-carboxylic acid tert-butyl ester
[0042]Under nitrogen protection, 160ml of n-butanol was added into the reaction flask, and the stirring was started. 20.0 g of compound II and 10.6 g of diisopropylethylamine were added. 9.0 g of propyl vinyl ether, 0.3 g of palladium acetate and 0.9 g of bis(2-phenylphosphorylphenyl)ether were added. The temperature was raised to 85-95° C., and after 4 hours of incubation, the reaction was detected by TLC. Add 200ml of n-heptane. Cool down to 5-15°C, keep warm and crystallize for 2.5h; filter with suction and wash with a mixed solution of n-butanol and n-heptane. After drying at 75°C for 5 h, 18.2 g of compound I was obtained, with a yield of 90.2% and a purity of 99.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com