Method for synthesizing 1,1,2,2-tetrafluoroethane
A technology of HCFC-22 and mixed gas, which is applied in chemical instruments and methods, catalyst carriers, organic chemistry, etc., can solve the problems of affecting the service life of the catalyst, difficulty in regeneration, and reduction of the specific surface area of the catalyst, so as to be suitable for technological production, Easy to regenerate and recycle, the effect of temperature stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0019] In the present invention, HCFC-22 pyrolysis generates unreacted HCFC-22 in the mixed gas product as an inert component (the inert component can also be any gas that does not react with TFE and hydrogen under reaction conditions such as nitrogen, Including the HCFC-22 gas that feeds in simultaneously with the TFE gas), take away the heat generated by the reaction, and can keep the catalytic reaction stably carried out. The synthesis method includes the following steps:
[0020] Weigh a certain amount of active catalyst and dissolve it in dilute acid, add a certain amount of carrier, soak for a period of time to remove water, and then dry the catalyst for use.
[0021] Load the catalyst into the reactor, N 2 After further removal of water under conditions, the H 2 Activate the catalyst, then pass the mixed gas containing TFE and H 2 The reaction was carried out to obtain the target object HFC-134.
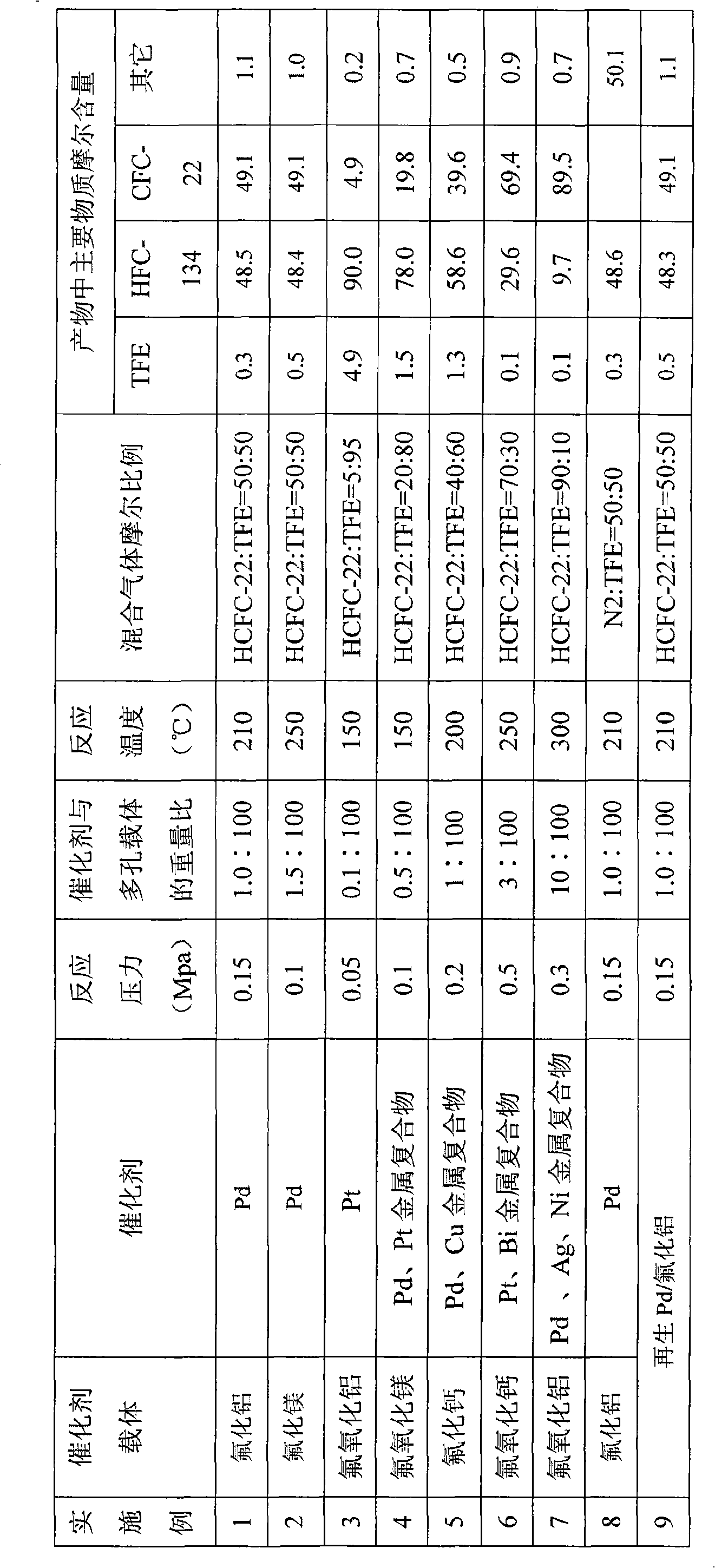
[0022] Experimental implementation 1-9 describes the present invention...
Embodiment 1
[0024] Catalyst preparation: wash 100 g of carrier aluminum fluoride with distilled water and dry in an oven at 120°C for 2 hours. Dissolve 1.7 g of the weighed active ingredient palladium chloride with dilute hydrochloric acid, then add the dried carrier and let stand overnight. After standing still, treat with a rotary evaporator, adjust the rotary evaporator to evaporate to dryness under reduced pressure at 80°C, 0.3atm, 120r / min, and store in a dry place.
[0025] Activation: Load the catalyst prepared in the previous step into the reactor, adjust the nitrogen flow rate to 30ml / min, raise the temperature to 250°C, and keep it for 2h. Then pass H2 and N2 mixed gas, keep 4h.
[0026] Reaction: After the catalyst activation is completed, the reaction temperature is adjusted to 210° C., and 200 ml / min of mixed gas of HCFC-22 and TFE (the molar ratio is 50:50) and 200 ml / min of hydrogen are introduced. After the reaction, the gas is washed with water, washed with alkali and t...
Embodiment 2
[0028] Catalyst preparation: Wash 100 g of carrier magnesium fluoride with distilled water and dry in an oven at 120°C for 2 hours. Dissolve 2.6 g of the weighed active ingredient palladium chloride with dilute hydrochloric acid, then add the dried carrier and let stand overnight. After standing still, treat with a rotary evaporator, adjust the rotary evaporator to evaporate to dryness under reduced pressure at 80°C, 0.3atm, 120r / min, and store in a dry place.
[0029] Catalyst activation was as the catalyst activation process in Example 1.
[0030] Reaction: After the catalyst activation is completed, the reaction temperature is adjusted to 250° C., and 200 ml / min of mixed gas of HCFC-22 and TFE (the molar ratio is 50:50) and 200 ml / min of hydrogen are introduced. After the reaction, the gas is washed with water, washed with alkali and then dried, and detected by gas chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com