Imatinib mesylate polymorph and pharmaceutical composition
A technology of imatinib mesylate and polymorphic form, which can be used in drug combinations, antipyretics, anti-infectives, etc., can solve problems such as complicated processes, poor product purity, and unsuitability for industrial production.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] The preparation of embodiment 1 polymorph I
[0103] plan 1,
[0104] Add 500.0 g of imatinib mesylate into the reaction flask, add 2500 ml of dimethylformamide, raise the temperature to 60°C, and stir to dissolve. 12.5 L of acetone was added, and the crystal was grown at 60° C. for 2 hours. After cooling down to room temperature naturally, refrigerated crystallization overnight. Suction filtration, filter cake rinse with a small amount of acetone. The filter cake was dried under reduced pressure at 50°C and -0.095MPa, and was assisted with phosphorus pentoxide. 445.1 g of light yellow solid was obtained. Yield: 89.0%, purity 99.92%
[0105]
[0106] Note: Imalic acid: 4-(4-methylpiperazinemethyl)-benzoic acid
[0107] Imamamine: N-(5-amino-2-methylphenyl)-4-(3-pyridine)-pyrimidinamine
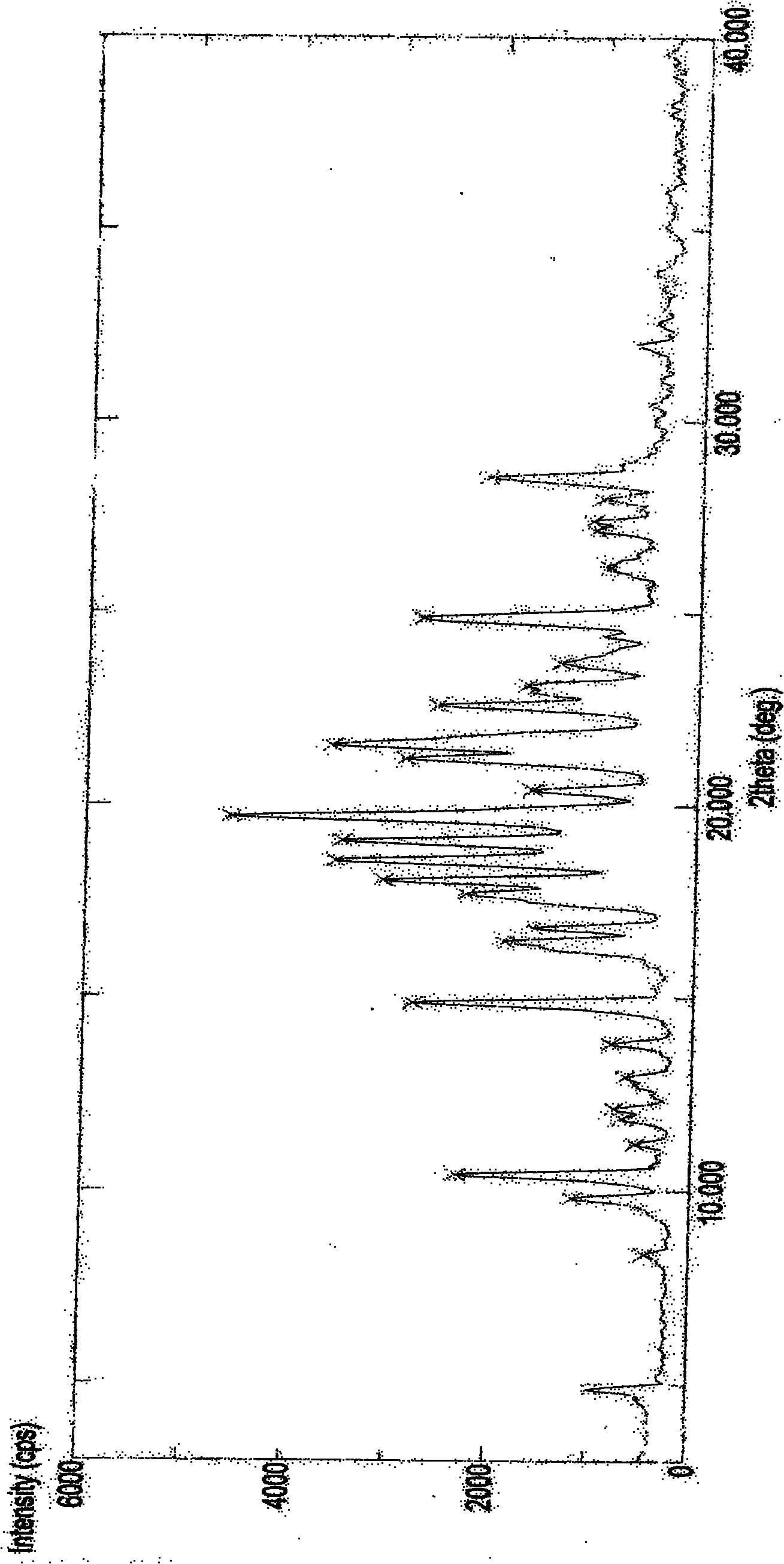
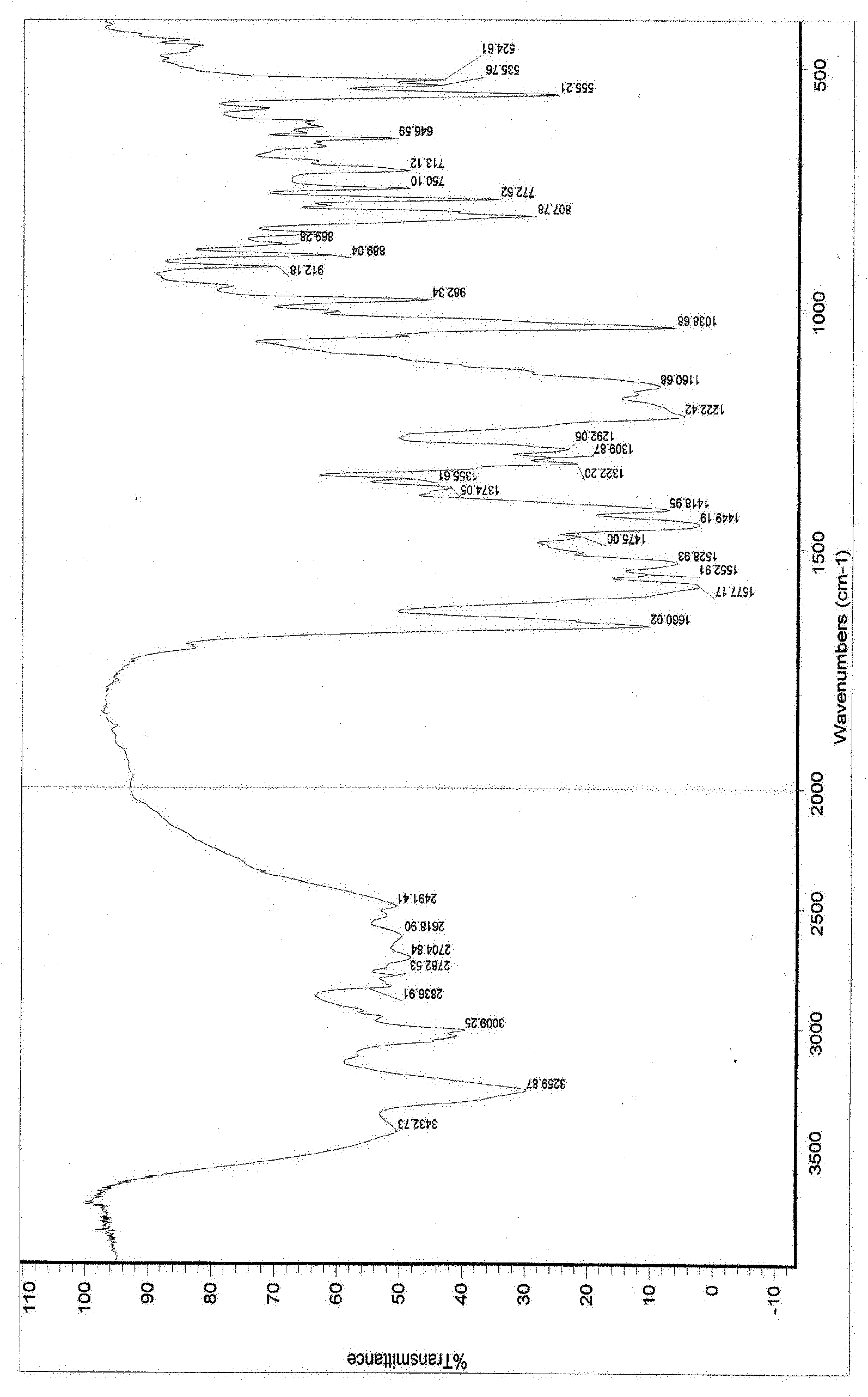
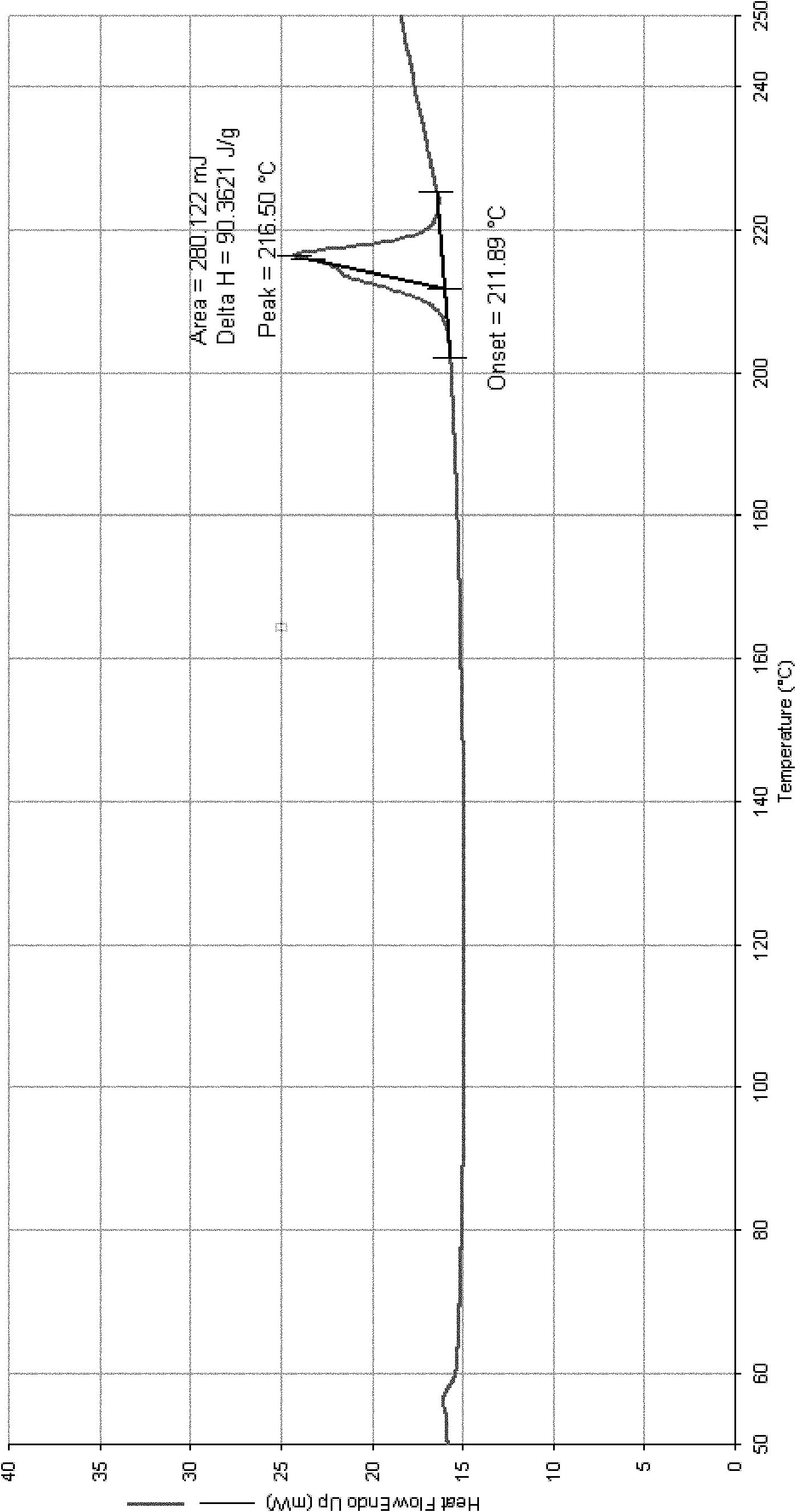
[0108] The resulting product of scheme 1 has been detected as follows: XRPD detection (see figure 1 ); IR detection (see figure 2 ); DSC detection (see image 3 ); TGA (se...
Embodiment 2
[0116] The product obtained in scheme 3 has been detected as follows: XRPD detection (see Figure 5 ). The prescription and preparation technology of embodiment 2 imatinib mesylate tablet:
[0117] The above-mentioned imatinib mesylate polymorph I was formulated into tablets containing 100 mg with several excipients as follows.
[0118] Imatinib mesylate I (calculated as imatinib)
100g
170g
6g
Crospovidone
20g
Micropowder silica gel
2g
2g
suppress
1000 pieces
[0119] Weigh the microcrystalline cellulose, hypromellose and crospovidone according to the prescription, mix them uniformly to obtain auxiliary material powder, and set aside. Weigh the imatinib mesylate according to the prescription, and mix it evenly with the auxiliary material powder to obtain the drug-containing mixed powder. Add appropriate amount of water to the...
Embodiment 3
[0122] The prescription and preparation technology of embodiment 3 imatinib mesylate capsules:
[0123] The above-mentioned imatinib mesylate polymorph I was formulated into tablets containing 100 mg with several excipients as follows.
[0124] Imatinib mesylate I (calculated as imatinib)
100g
240g
Crospovidone
50g
Micropowder silica gel
5g
5g
filling
1000 capsules
[0125] Weigh microcrystalline cellulose, crospovidone, micropowder silica gel and magnesium stearate according to the prescription, and mix them uniformly to obtain auxiliary material powder. Weigh the imatinib mesylate according to the prescription, and mix it evenly with the auxiliary material powder to obtain the drug-containing mixed powder. The drug-containing mixed powder is filled into No. 00 hard capsules to prepare capsules.
[0126] Capsules containing 400 mg of imatinib can be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com