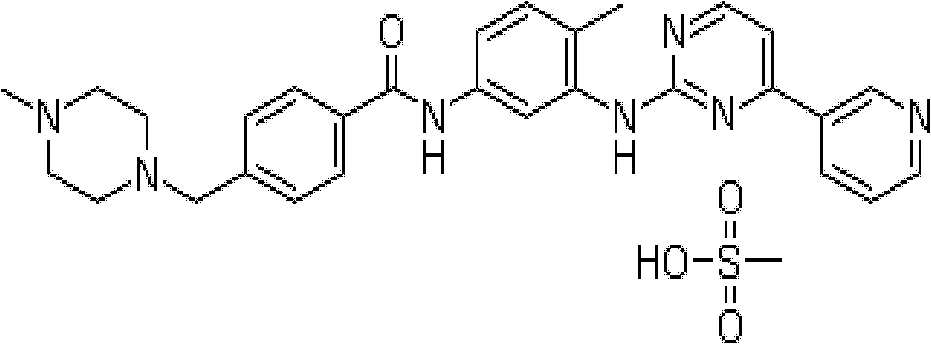
Methanesulfonic acid imatinib polymorphic substance and medical combination thereof
A technology of imatinib mesylate and polymorphs, which is applied in drug combinations, antineoplastic drugs, pharmaceutical formulations, etc., can solve the problems of inability to effectively remove mechanical impurities, unstable reproducibility, and poor product purity, etc. problem, to achieve the effect suitable for industrial scale preparation, good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
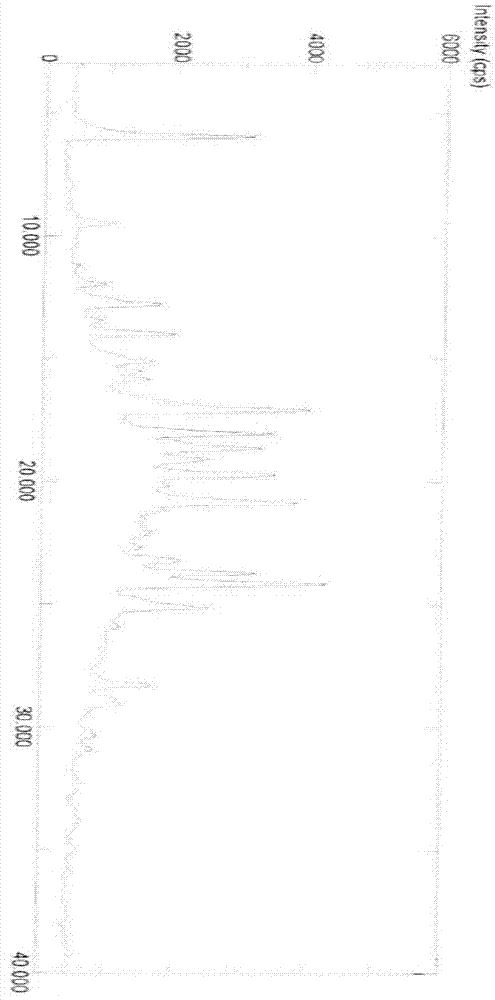
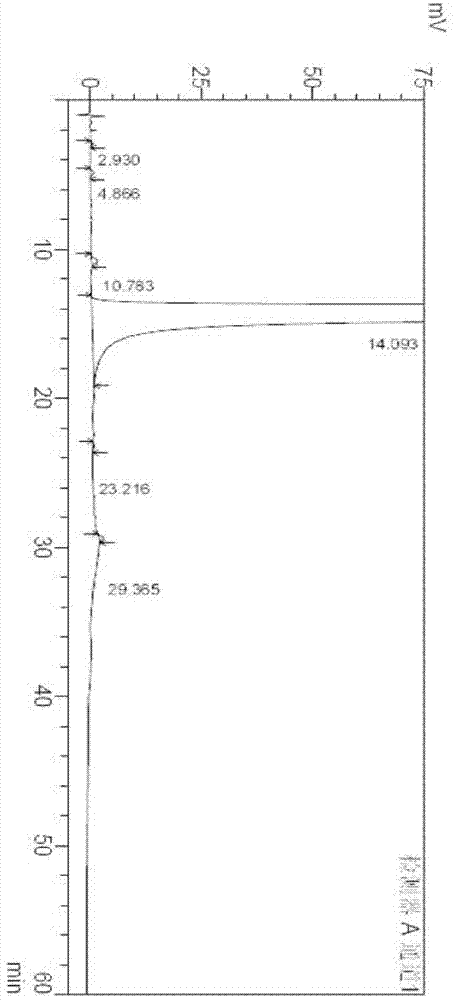
Image
Examples
Embodiment 1
[0101] Example 1 Preparation of crude imatinib mesylate
[0102] Imatinib (2Kg), methanesulfonic acid (390g) and acetone (20L) were added to the reaction flask, and the temperature was raised to reflux for 2.5 hours while stirring. It was cooled to room temperature, and stirred for 3 hours to crystallize. The filter cake was collected by suction filtration and dried under reduced pressure at 70°C to a constant weight to obtain 2.2 Kg of crude imatinib mesylate. The yield was 92.1%.
[0103] Purity: 98.7%.
Embodiment 2
[0104] Example 2 Preparation of Polymorph III
[0105] Preparation of imatinib free base
[0106] The crude imatinib mesylate (1.5Kg) obtained in Example 1 was added to water (15L), the temperature was raised to 60°C, and the mixture was stirred and dissolved. Suction to remove impurities. The filtrate was cooled to room temperature, and acetone (4.5L) was added with stirring. Sodium bicarbonate (214g) was added and crystallized at room temperature for 3 hours. After suction filtration, the filter cake was washed with water and dried to a constant weight to obtain 1.17Kg of imatinib. Yield: 92.9%.
[0107] Preparation of imatinib mesylate
[0108] The imatinib (80g), methanesulfonic acid (15.6g) and methyl tert-butyl ether (800ml) obtained above were added to the reaction flask, and the temperature was raised to reflux for 2 hours under stirring. It was cooled to room temperature and stirred for 1 hour to crystallize. The filter cake was collected by suction filtration and dried...
Embodiment 3
[0111] Example 3 Preparation of polymorph III
[0112] According to the existing method, we use different crystal forms of imatinib mesylate prepared by different processes as raw materials, and the data table of the preparation of polymorph III according to the scheme of Example 2 is as follows:
[0113]
[0114] It can be seen from the above test data that any crystal form of crude imatinib mesylate can be transformed into the crystal form of the present invention. At the same time, impurities can be effectively removed to obtain high purity imatinib mesylate up to 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com