Pharmaceutical composition of ornithine aspartate
A technology of ornithine aspartate and composition, which is applied in the direction of drug combination, pharmaceutical formula, medical preparations of non-effective ingredients, etc., can solve the problems of high content of excipients, difficulty in taking medicine, complicated process, etc., and achieve small dosage , stable blood drug concentration, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
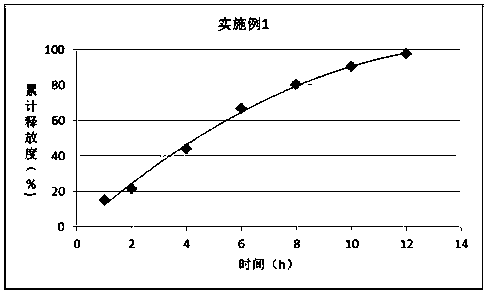
Embodiment 1
[0019] prescription:
[0020] Material composition
Prescription ratio (g)
Ornithine Aspartate
100
30
PEG6000
10
[0021] Preparation:
[0022] 1) Mix PLGA with PEG 6000 and heat to 55°C; 2) Add ornithine aspartic acid to disperse evenly;
[0023] 3) Cool to 25°C and crush to obtain.
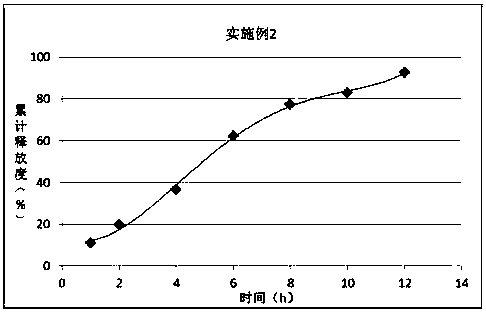
Embodiment 2
[0025] prescription:
[0026] Material composition
Prescription ratio (g)
100
PLGA65:35
60
PEG6000
30
[0027] Preparation:
[0028] 1) Mix PLGA with PEG 6000 and heat to 38°C; 2) Add ornithine aspartic acid to disperse evenly;
[0029] 3) Cool to 30°C and crush to obtain.
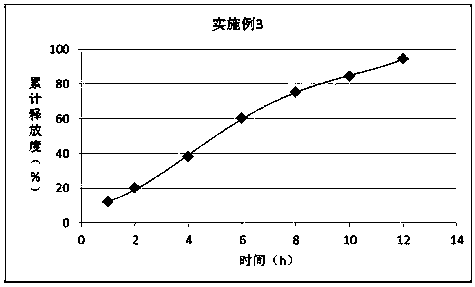
Embodiment 3
[0031] prescription:
[0032] Material composition
Prescription ratio (g)
100
PLGA50:50
50
PEG6000
15
[0033] Preparation:
[0034] 1) Mix PLGA with PEG 6000 and heat to 45°C; 2) Add ornithine aspartic acid to disperse evenly;
[0035] 3) Cool to 28°C and crush to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com