Synthetic method of pitavastatin tert-butyl ester
A technology of pitavastatin tert-butyl ester and a synthesis method, which is applied in the field of synthesis of pitavastatin tert-butyl ester, can solve the problems of unsuitability for industrial production, incomplete oxidation of intermediates, poor stereoselectivity, etc., and achieves small steric hindrance. , the reaction conditions are mild and controllable, and the stereoselectivity is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
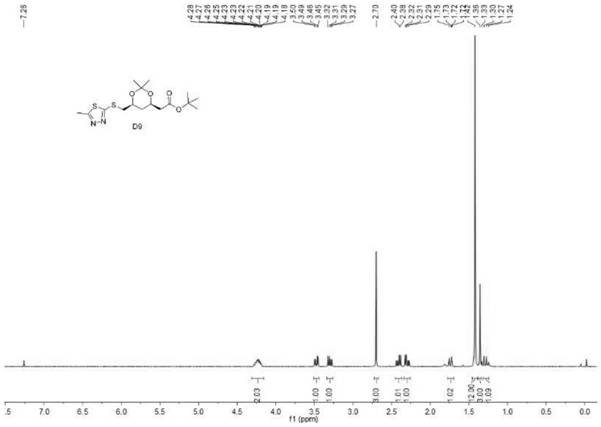
[0052] Embodiment 1 (R is a methyl group)
[0053] Under nitrogen protection, 160g (1.5mol, 3.0eq) of sodium carbonate, (4R-Cis)-6-chloromethyl-2,2-dimethyl-1,3- Dioxolane-4-acetic acid tert-butyl ester 139.5g (0.5mol, 1.0eq), 1,4-dioxane 700g, 2-mercapto 5-methyl 1,3,4-thiadiazole 79g (0.6 mol, 1.2eq) to start stirring, slowly raise the temperature to 80°C, stir and react for 6h, take a sample for TLC (developing solvent: petroleum ether: ethyl acetate = 1:1v / v) to detect the complete conversion of raw materials, then concentrate under reduced pressure to remove the solvent 1 , 4-dioxane, add 500mL of water, 500mL of ethyl acetate, continue to stir for 15 minutes, then stand for layering, separate the organic phase, extract the water phase with 500mL of ethyl acetate x 2, and combine the organic phases. Concentrate to brown-black oily substance B
[0054] 195g (with a small amount of solvent), the purity is 93.7%. Not purified, directly used in the next oxidation reaction;...
Embodiment 2
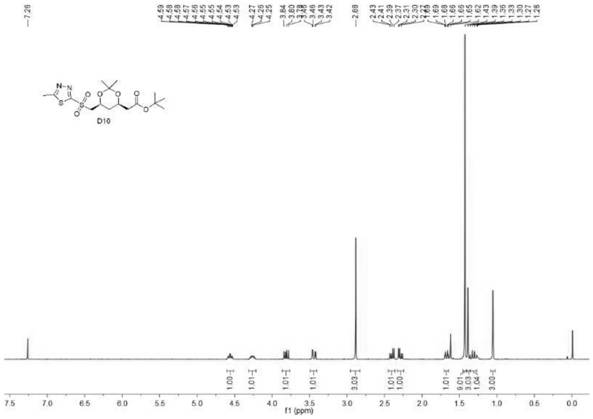
[0059] Embodiment 2 (R is phenyl)
[0060] Under nitrogen protection, 207g (1.5mol, 3.0eq) of potassium carbonate, (4R-Cis)-6-chloromethyl-2,2-dimethyl-1,3- Dioxolane-4-acetic acid tert-butyl ester 139.5g (0.5mol, 1.0eq), N,N-dimethylformamide 700g, 2-mercapto 5-phenyl 1,3,4-thiadiazole 116g ( 0.6mol, 1.2eq), start stirring, slowly raise the temperature to 80°C, stir and react for 6 hours, take a sample for TLC (developing solvent: petroleum ether: ethyl acetate = 1:1v / v) to detect the complete conversion of raw materials, then concentrate under reduced pressure to remove the solvent N,N-Dimethylformamide, add 500mL of water, 500mL of ethyl acetate, continue to stir for 15 minutes, then stand for layering, separate the organic phase, extract the water phase with 500mL of ethyl acetate*2, and combine the organic phases. Concentration gave 225 g of substance B (with a small amount of solvent) in a brownish-black oily substance with a purity of 94.7%. Not purified, directly use...
Embodiment 3
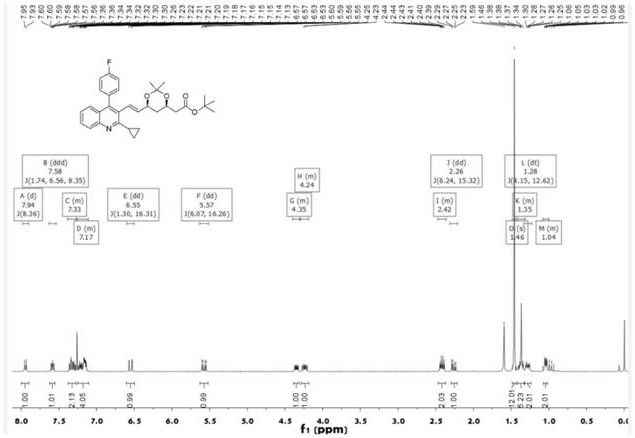
[0065] The substituent R of substance A is cyclopropyl, and other conditions and charging ratio are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com