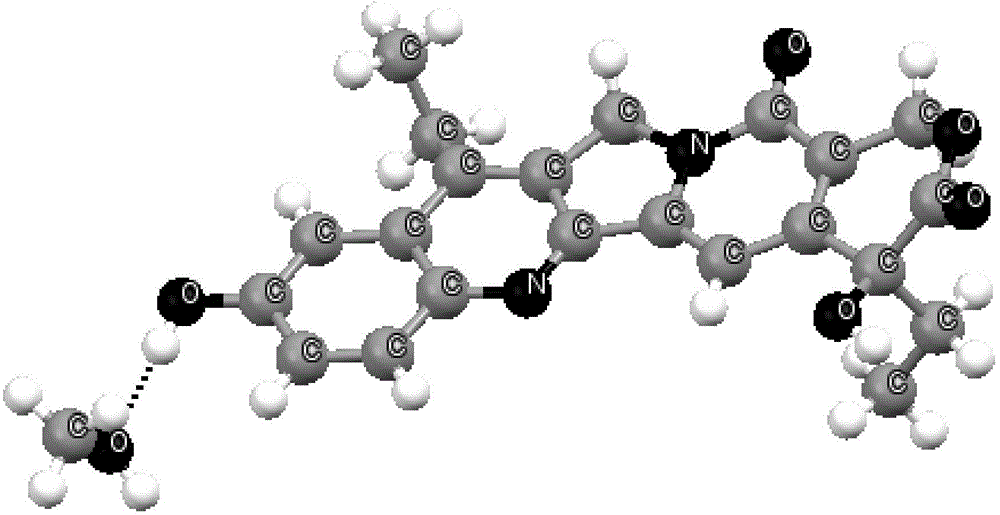
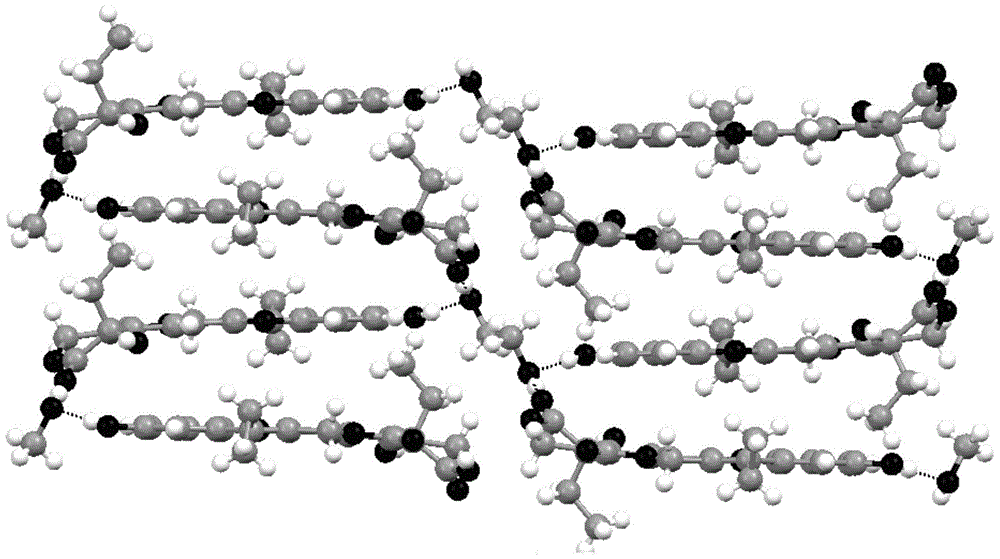
7-ethyl-10-hydroxycamptothecine crystal forms, and preparation method and application thereof
A technology of hydroxycamptothecin and crystal form, applied in the field of medicinal chemistry, can solve the problem of low solubility of polymorphs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
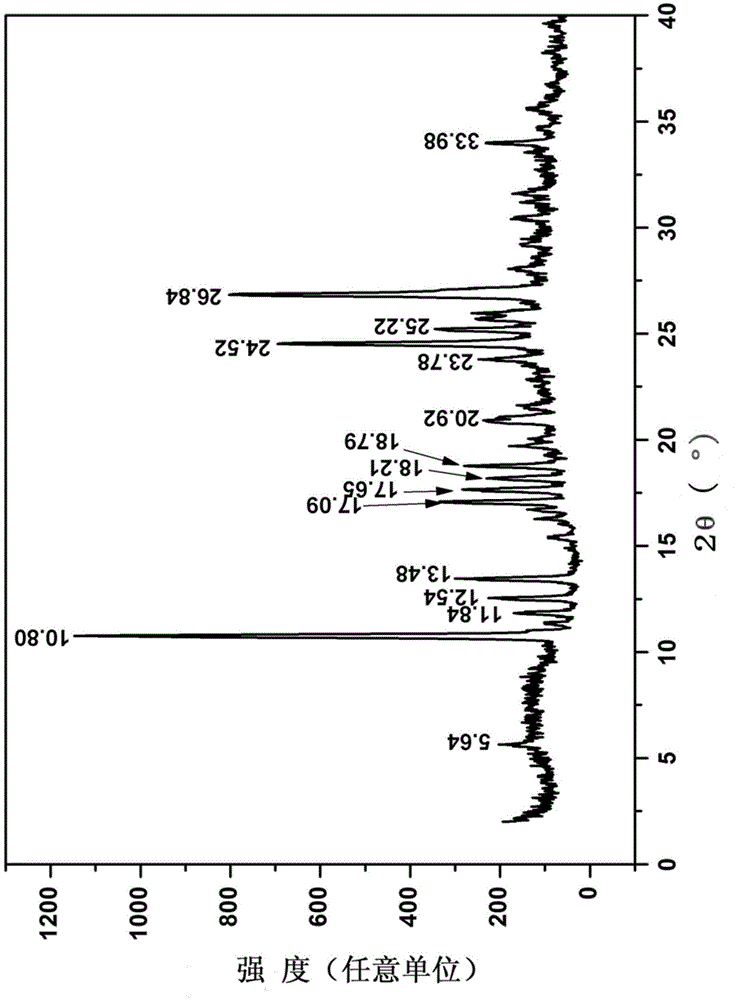
[0057] Examples 1-5 are the preparation of crystalline form II of 7-ethyl-10-hydroxycamptothecin, and examples 6-10 are the preparation of crystalline form III of 7-ethyl-10-hydroxycamptothecin.
[0058] Embodiment 1, the preparation of 7-ethyl-10-hydroxycamptothecin crystal form II
[0059] Weigh 50mg of 7-ethyl-10-hydroxycamptothecin into a reaction bottle, add 20ml of a mixed solvent of methanol: chloroform with a volume ratio of 1:1, stir to dissolve, evaporate the solvent at 10-20°C, and separate by filtration Solid, the solid was vacuum-dried at 50°C to constant weight to obtain 7-ethyl-10-hydroxycamptothecin crystal form II.
Embodiment 2
[0060] Example 2, Preparation of 7-ethyl-10-hydroxycamptothecin crystal form II
[0061] Weigh 30mg of 7-ethyl-10-hydroxycamptothecin into a reaction flask, add 210ml of methanol solvent, stir to dissolve, evaporate the solvent at 10-20°C, filter and separate the solid, and dry the solid in vacuum at 50°C until constant Weight, that is, 7-ethyl-10-hydroxycamptothecin crystal form II.
Embodiment 3
[0062] Example 3, Preparation of 7-ethyl-10-hydroxycamptothecin crystal form II
[0063] Weigh 50mg of 7-ethyl-10-hydroxycamptothecin into a reaction flask, add 12ml of methanol:chloroform mixed solvent with a volume ratio of 1:1, stir at room temperature, filter and separate the solid, and dry the solid under vacuum at 50°C To constant weight, the crystal form II of 7-ethyl-10-hydroxycamptothecin was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com