Novel crystal form of neratinib maleate and preparation method thereof
A technology of maleate salt and maleic acid, which is applied in the field of chemical medicine, can solve the problems of unfavorable long-term storage, etc., and achieve the effects of increasing the dissolution rate, avoiding crystal transformation, and avoiding solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The preparation method of formula (I) compound maleate crystal form A:
[0080] 101.2 mg of the compound of formula (I) was dissolved in 4 mL of dichloromethane, 73.3 mg of maleic acid was added, and the reaction was stirred at room temperature for 12 hours. The solid was collected by centrifugation and obtained.
[0081] The maleate product prepared by the above-mentioned method, its 1 The HNMR identification data are as follows, and the data show that the molar ratio of the compound of formula (I) to maleic acid is 1:3.
[0082] 1 HNMR(400MHz,DMSO-d6)δ9.81(s,1H),9.76(s,1H),8.95(s,1H),8.60(d,J=4.1Hz,1H),8.54(s,1H), 7.88(td, J1=7.7Hz, J2=1.7Hz, 1H), 7.59(d, J=7.8Hz, 1H), 7.37-7.42(m, 3H), 7.21-7.26(m, 2H), 6.82–6.70 (m,2H),6.20(s,6H),5.29(s,2H),4.33(q,J=6.9Hz,2H),3.96(d,J=5.4Hz,2H),2.81(s,6H) , 1.47 (t, J=6.9Hz, 3H).
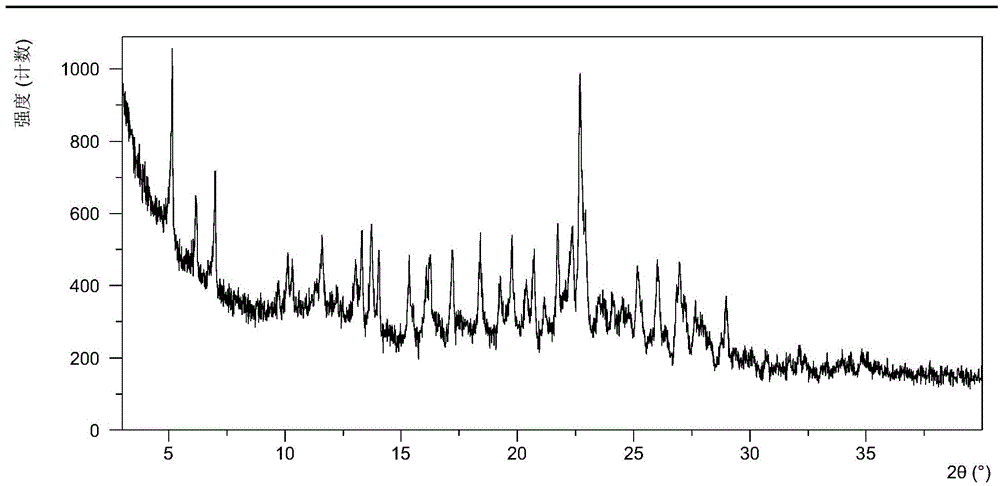
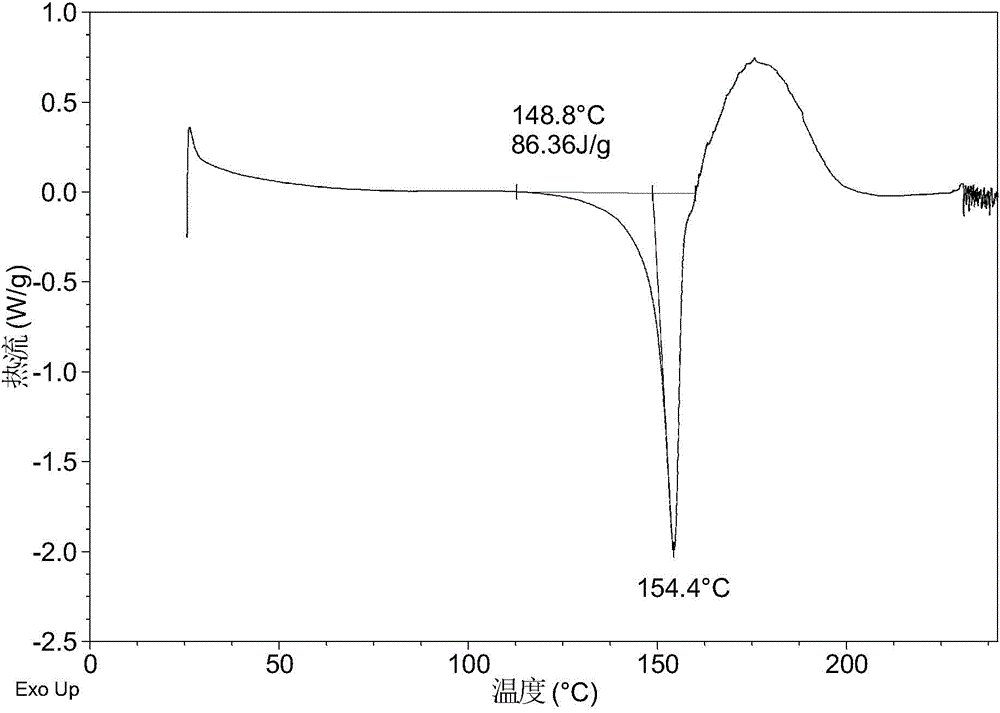
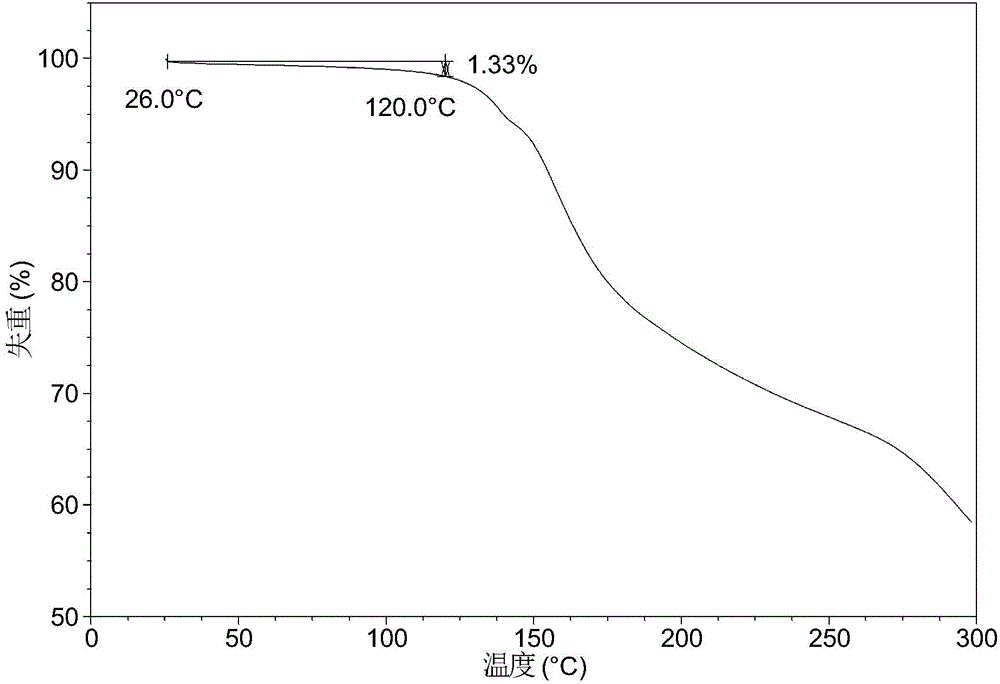
[0083] After testing, the solid obtained in this example is crystal form A, and its X-ray powder diffraction data are shown in Table 1. Its XRPD p...
Embodiment 2
[0088] The preparation method of formula (I) compound maleate crystal form A:
[0089] 19.8 mg of the compound of formula (I) was added to 1 mL of acetone to prepare a suspension, 13.0 mg of maleic acid was added, and the reaction was stirred for 12 hours at room temperature. The solid was collected by centrifugation and obtained.
[0090] After testing, the solid obtained in this example is crystal form A, and its X-ray powder diffraction data are shown in Table 2.
[0091] Table 2
[0092] 2theta
[0093] 13.94
Embodiment 3
[0095] The preparation method of formula (I) compound maleate crystal form A:
[0096] Add 10.0 mg of the compound of formula (I) to 0.5 mL of isopropanol to make a suspension, add 6.0 mg of maleic acid, add n-hexane dropwise to 0.6 mL while stirring, after the dropwise addition is complete, stir the reaction at room temperature 12 hours. The solid was collected by centrifugation and obtained.
[0097] After testing, the solid obtained in this example is crystal form A, and its X-ray powder diffraction data are shown in Table 3.
[0098] table 3
[0099] 2theta
[0100] 12.25
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com