Crystalline form of butlutamide and preparation method thereof
A technology of bulutamide and crystal form, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulations, organic chemistry, etc., can solve the problem of not providing information on the crystal form of bulutamide, etc., and achieves easy storage and processing. , good processability, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
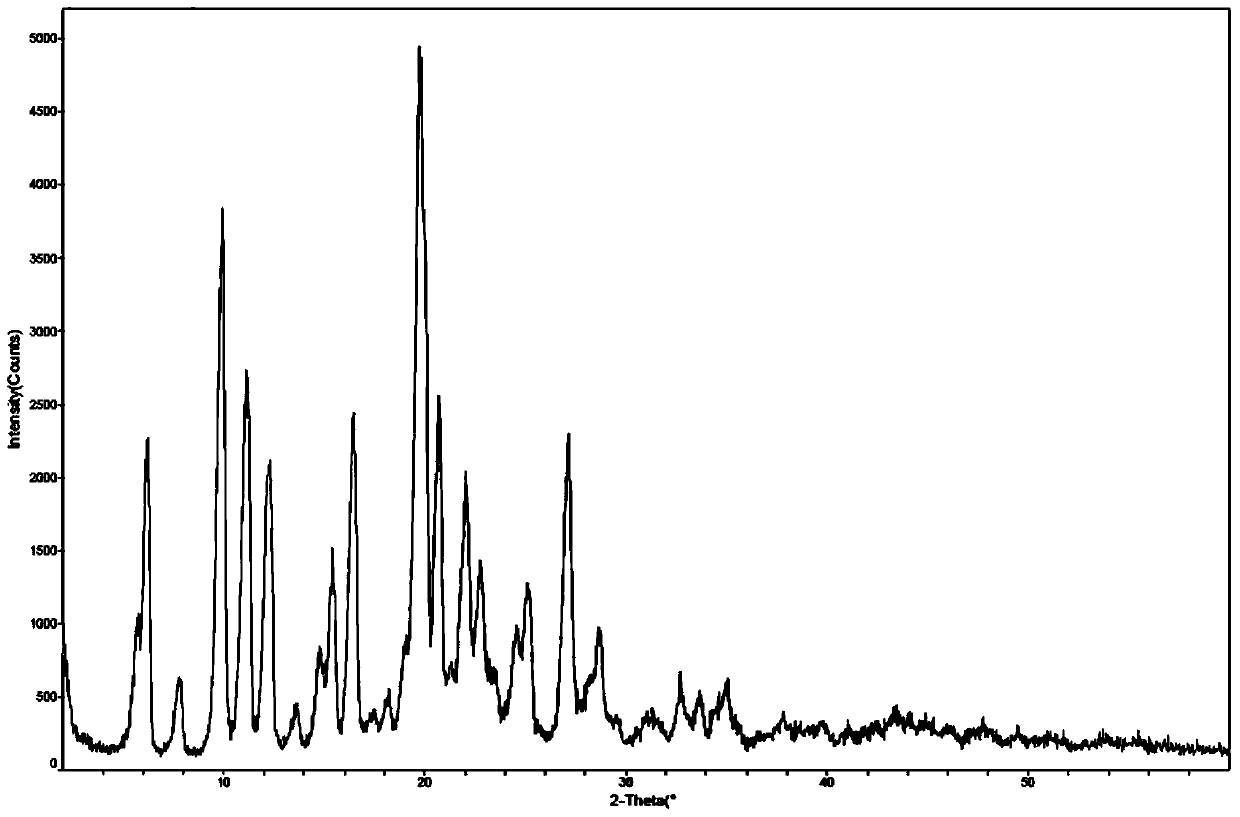
[0044] Example 1: Preparation of bulutamide β-crystal form
[0045] The preparation method of bulutamide refers to N-methyl-5-[3-(4-cyano-3-trifluoromethylphenyl)-5,5-dimethyl-4-oxo in Example 41 of CN104341351 -2-thiotetrahydroimidazol-1-yl-butyl]-3-fluorobenzamide, namely the method of bulutamide, to obtain bulutamide.
[0046] At 25°C, 5g of bulutamide was added to a mixed solvent of 48ml of acetone and water (2:1 v / v), the reaction system was heated to reflux, stirred for 15 minutes, cooled to room temperature naturally, and stirred at room temperature 6 hours.
[0047] After filtration, the filter cake was washed with 5 ml of acetone and water (2:1 v / v), and the filter cake was dried in a vacuum blast drying oven at 60°C for 8 hours to obtain 4.2 g of white needle crystals with a yield of 84%.
Embodiment 2
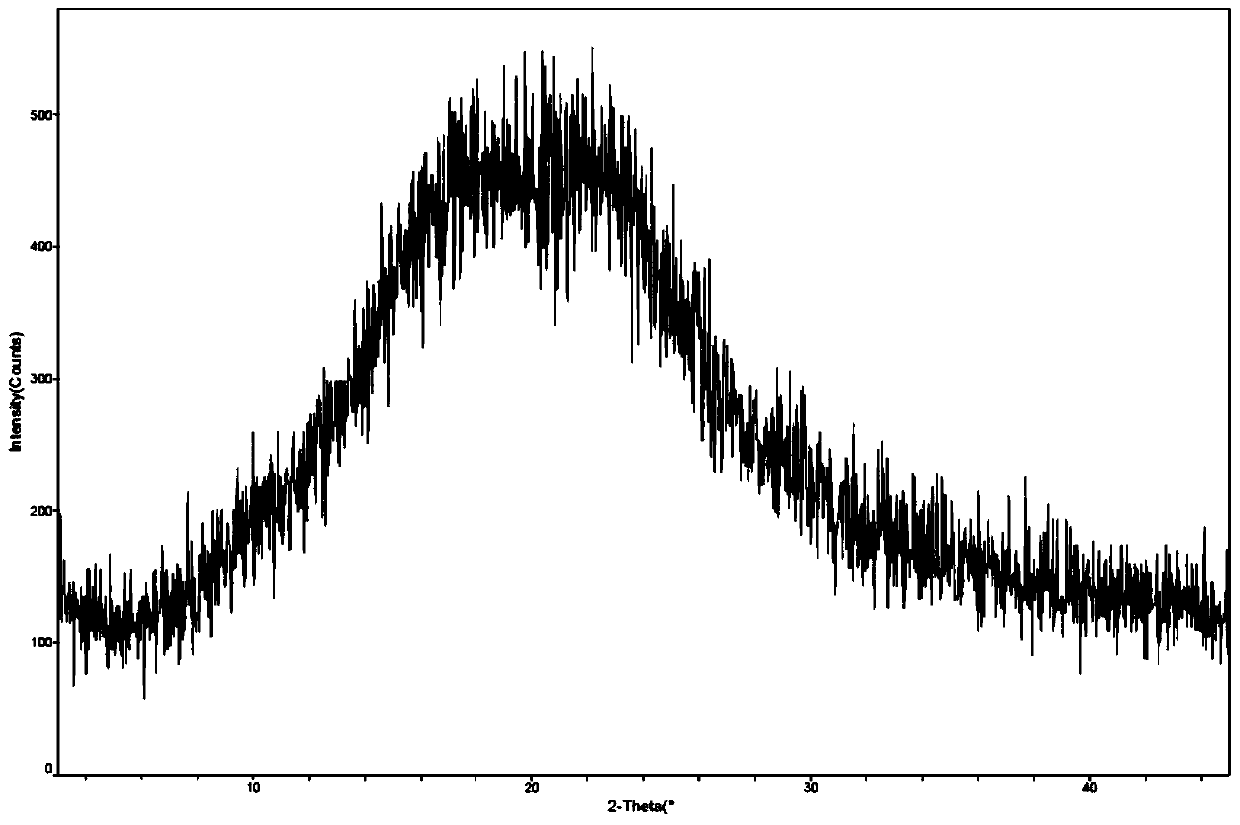
[0048] Example 2: Preparation of the amorphous form of bulutamide
[0049] Under the condition of 25°C, 5 g of bulutamide β-crystal form raw material was put into 60 ml of tetrahydrofuran, the reaction system was heated to reflux for 30 minutes, and stirred for 4 hours under the reflux state. The solution was cooled to 65°C and filtered while hot through a pad of celite.
[0050] The obtained filtrate was cooled to 50° C., under vigorous stirring, the filtrate was added to 100 ml of n-hexane within 20 minutes, and the stirring was continued at room temperature for 2 hours.
[0051] The suspension was filtered and the filter cake was dried under vacuum at 60°C to give 3.8 g of the title compound in 76% yield.
Embodiment 3
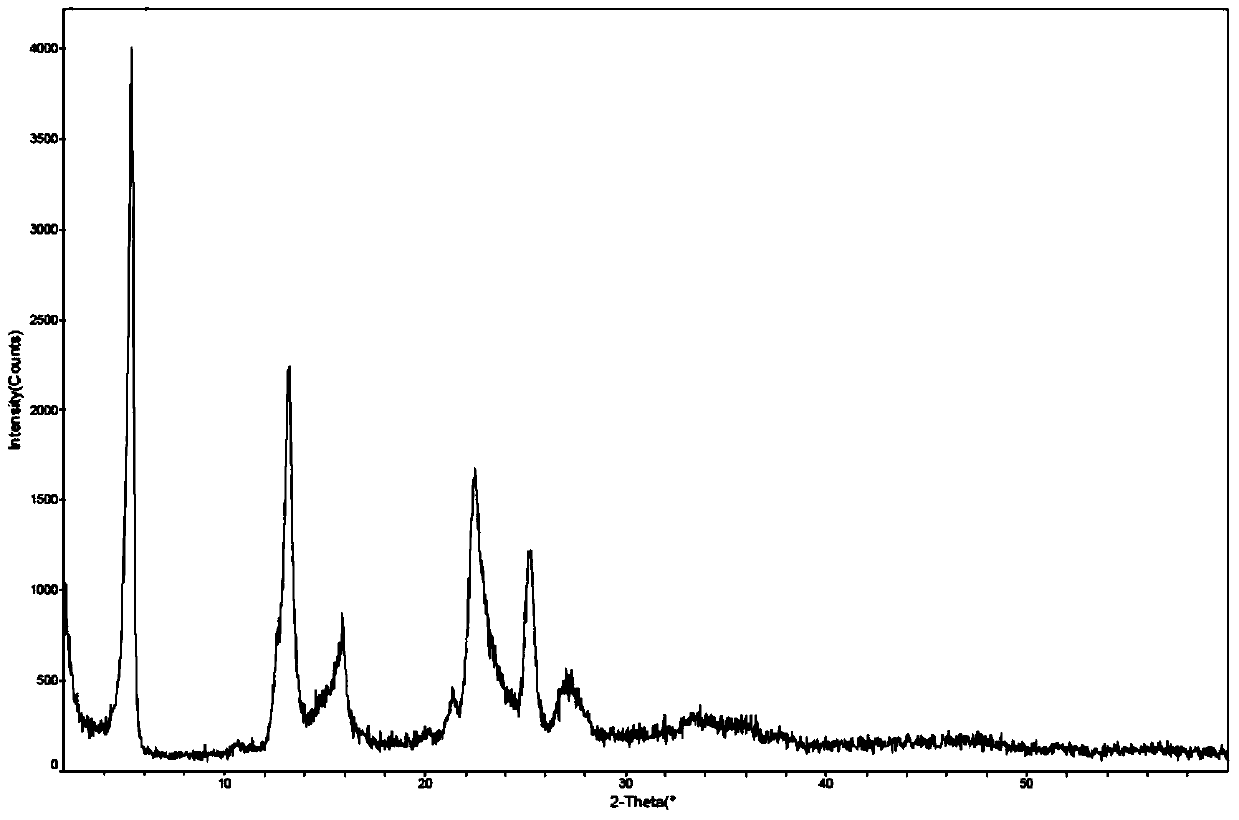
[0052] Example 3: Preparation of bulutamide α-crystal form
[0053] At 25°C, 5 g of bulutamide β-crystalline form or amorphous form was put into 55 ml of methyl tert-butyl ether solvent, the reaction system was heated to reflux for 30 minutes, and stirred for 2 hours under reflux. The reaction system was cooled to 65°C, filtered through a celite layer while hot, and the filter layer was washed with 8 ml of methyl tert-butyl ether.
[0054] The obtained filtrate was cooled to 45°C, and the stirring was continued for 2 hours. The obtained reaction system was rapidly cooled to 5° C. within 5 minutes, and kept stirring for 2 hours to produce off-white slurry, which was kept at 5-10° C. for 18 hours.
[0055]The suspension was filtered, the filter cake was washed with 5 ml of methyl tert-butyl ether, and the filter cake was dried in a vacuum blast drying oven at 60 °C for 8 hours to obtain 3.5 g of the title compound as a white crystalline powder, yield 70% .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com