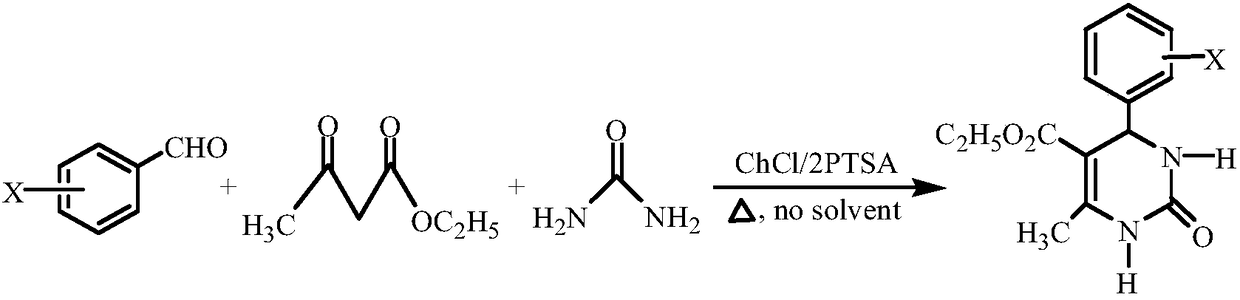
Method for synthesizing 4-halophenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydro-pyrimidin-2(1H)-one
A kind of technology of ethoxycarbonyl and halogenated phenyl, which is applied in the field of synthesis of 4-halogenated phenyl-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one It can solve the problems of complex catalyst preparation process, long reaction time, limited application, etc., and achieve the effect of reducing the use of organic solvents and corrosive catalysts, simple and convenient processing, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Reaction equation:
[0021]
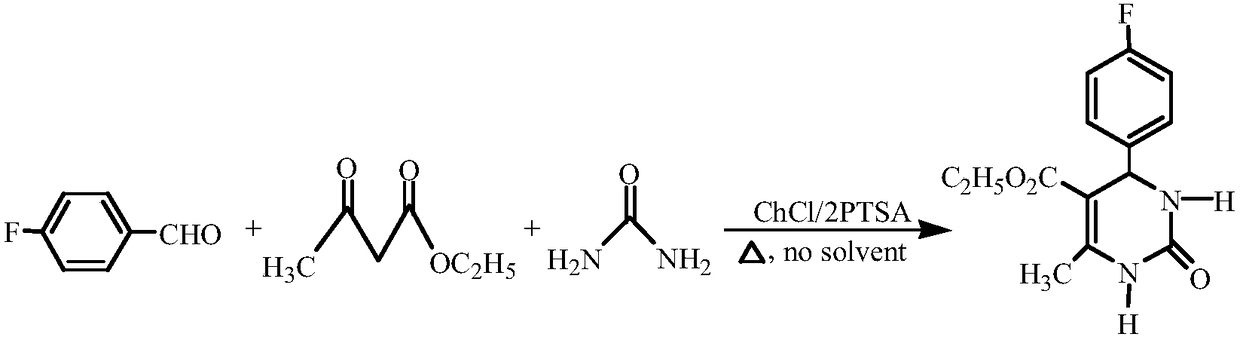
[0022] Experimental method: Add 0.6mmol of catalyst DES, 2mmol of 4-fluorobenzaldehyde, 2mmol of ethyl acetoacetate and 3mmol of urea into a 25mL round-bottomed flask, stir the reaction for 40min at 70°C and stop the reaction. After the reaction, cool to room temperature, add ice-water mixture to fully separate the product, filter with suction, and wash with distilled water for 3 times to obtain the crude product. Recrystallization from ethanol-water solution can give 4-(4-fluorophenyl)-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one with a yield of 91%.
Embodiment 2
[0024] Reaction equation:
[0025]
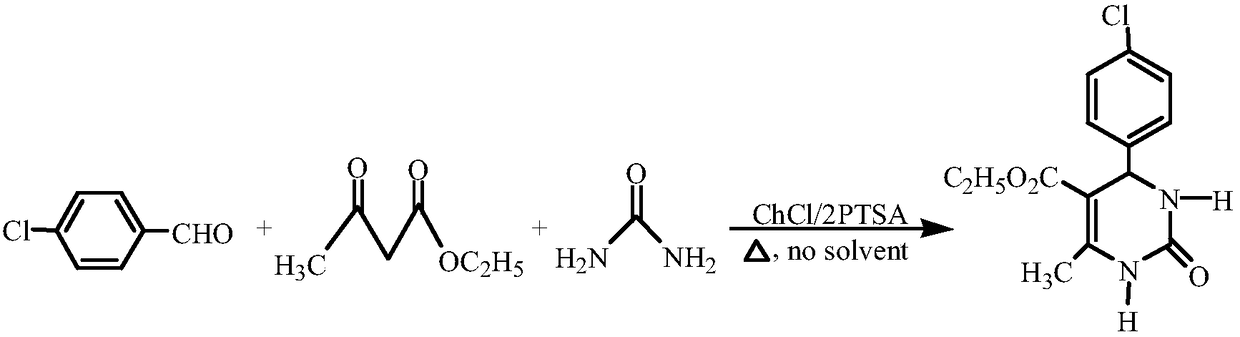
[0026] Experimental method: Add 0.6mmol of catalyst DES, 2mmol of 4-chlorobenzaldehyde, 2mmol of ethyl acetoacetate and 3mmol of urea into a 25mL round bottom flask, stir the reaction for 40min at 70°C and stop the reaction. After the reaction, cool to room temperature, add ice-water mixture to fully separate the product, filter with suction, and wash with distilled water for 3 times to obtain the crude product. Recrystallization with ethanol-water solution can give 4-(4-chlorophenyl)-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one with a yield of 92%.
Embodiment 3
[0028] Reaction equation:
[0029]
[0030] Experimental method: Add 0.6mmol of catalyst DES, 2mmol of 4-bromobenzaldehyde, 2mmol of ethyl acetoacetate and 3mmol of urea into a 25mL round bottom flask, stir the reaction for 40min at 70°C and stop the reaction. After the reaction, cool to room temperature, add ice-water mixture to fully separate the product, filter with suction, and wash with distilled water for 3 times to obtain the crude product. Recrystallization with ethanol-water solution can give 4-(4-bromophenyl)-5-ethoxycarbonyl-6-methyl-3,4-dihydropyrimidin-2(1H)-one with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com