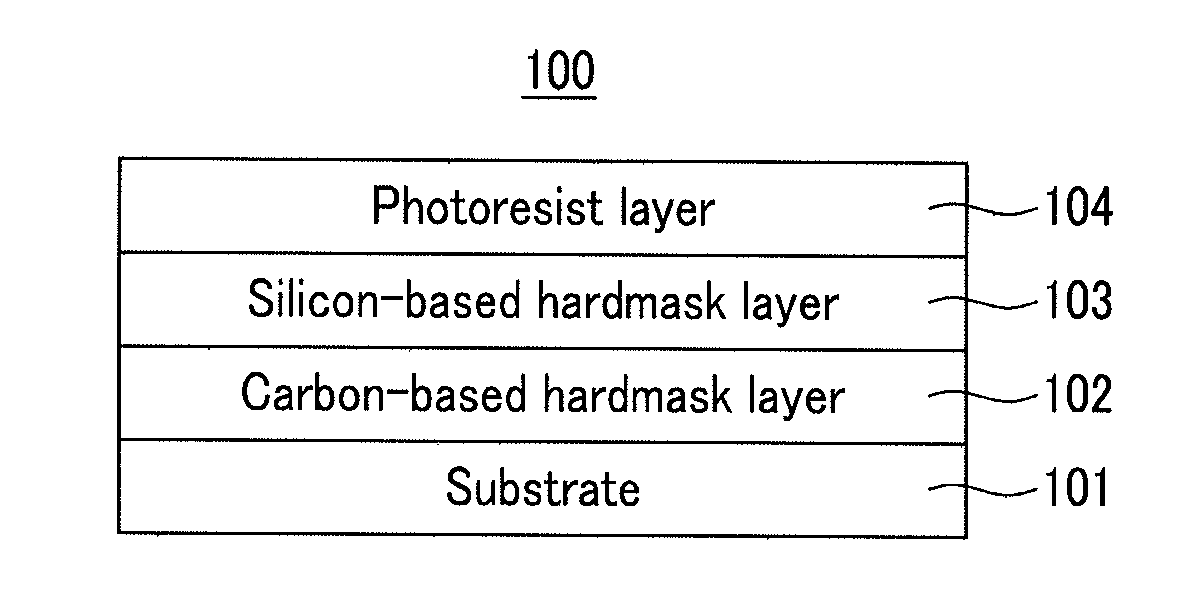
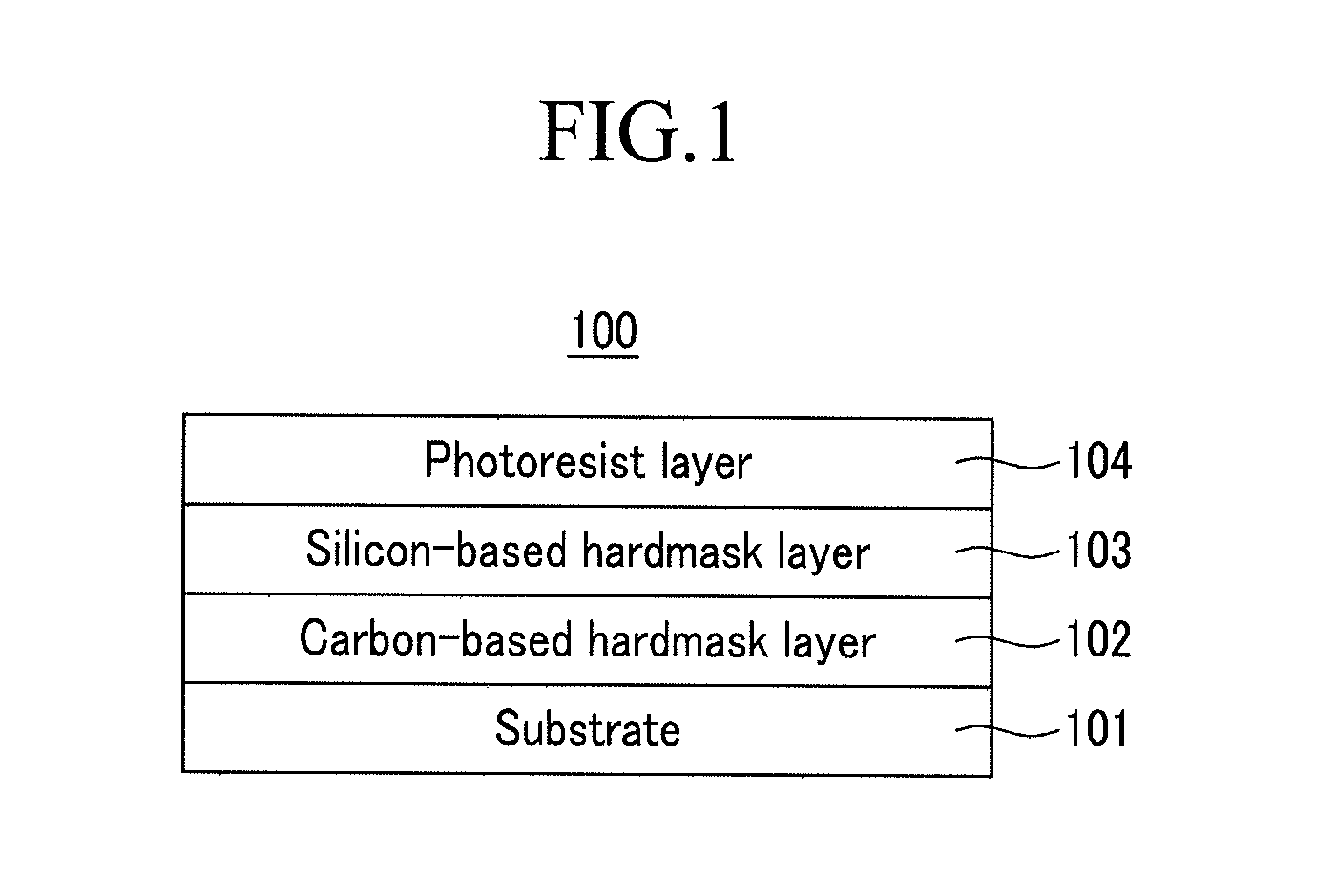
Hardmask composition for forming resist underlayer film, process for producing a semiconductor integrated circuit device, and semiconductor integrated circuit device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0096]1,750 g of methyltrimethoxysilane, 340 g of phenyltrimethoxysilane, and 313 g of trimethoxysilane were dissolved in 5,600 g of propylene glycol monomethyl ether acetate (PGMEA) in a 10-liter four-neck flask equipped with a mechanical agitator, a condenser, a dropping funnel, and a nitrogen inlet tube. To the solution was added 925 g of an aqueous nitric acid solution (1,000 ppm). After the mixture was allowed to react at 60° C. for 1 hour, methanol was removed from the reaction mixture under reduced pressure. The reaction was continued for 1 week while maintaining the reaction temperature at 50° C. After completion of the reaction, hexane was added to the reaction mixture to precipitate a polymer.
[0097]2.0 g of the polymer was diluted with 100 g of MIBK, and 0.002 g of pyridinium p-toluenesulfonate and 0.02 g of acetic anhydride were added thereto. A portion of the resulting solution was spin-coated on a silicon wafer coated with silicon nitride and a carbon-based hardmask, fo...
example 2
[0098]49.3 g of methyltrimethoxysilane, 43.9 g of phenyltrimethoxysilane, and 306.8 g of 1,2-bis(triethoxysilyl)ethane were dissolved in 1,600 g of propylene glycol monomethyl ether acetate (PGMEA) in a 3-liter four-neck flask equipped with a mechanical agitator, a condenser, a dropping funnel, and a nitrogen inlet tube. To the solution was added 131.3 g of an aqueous nitric acid solution (1,000 ppm). After the mixture was allowed to react at room temperature for 1 hour, alcohols were removed from the reaction mixture under reduced pressure. The reaction was continued for 1 week while maintaining the reaction temperature at 50° C. After completion of the reaction, hexane was added to the reaction mixture to precipitate a polymer.
[0099]2.0 g of the polymer was diluted with 100 g of MIBK, and 0.002 g of pyridinium p-toluenesulfonate and 10 g of propylene glycol propyl ether were added thereto. A portion of the resulting solution was spin-coated on a silicon wafer coated with silicon n...
example 3
[0100]220.1 g of methyltrimethoxysilane, 68.0 g of phenyltrimethoxysilane and 612.0 g of tetraethyl orthosilicate were dissolved in 2,100 g of propylene glycol monomethyl ether acetate (PGMEA) in a 5-liter four-neck flask equipped with a mechanical agitator, a condenser, a dropping funnel and a nitrogen inlet tube. To the solution was added 222.3 g of an aqueous nitric acid solution (1,000 ppm). After the mixture was allowed to react at room temperature for 5 hours, alcohols were removed from the reaction mixture under reduced pressure. The reaction was continued for 1 week while maintaining the reaction temperature at 50° C. After completion of the reaction, hexane was added to the reaction mixture to precipitate a polymer.
[0101]2.0 g of the polymer was diluted with 100 g of MIBK, and 0.002 g of pyridinium p-toluenesulfonate and 0.02 g of phenyltrimethoxysilane were added thereto. A portion of the resulting solution was spin-coated on a silicon wafer coated with silicon nitride and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com