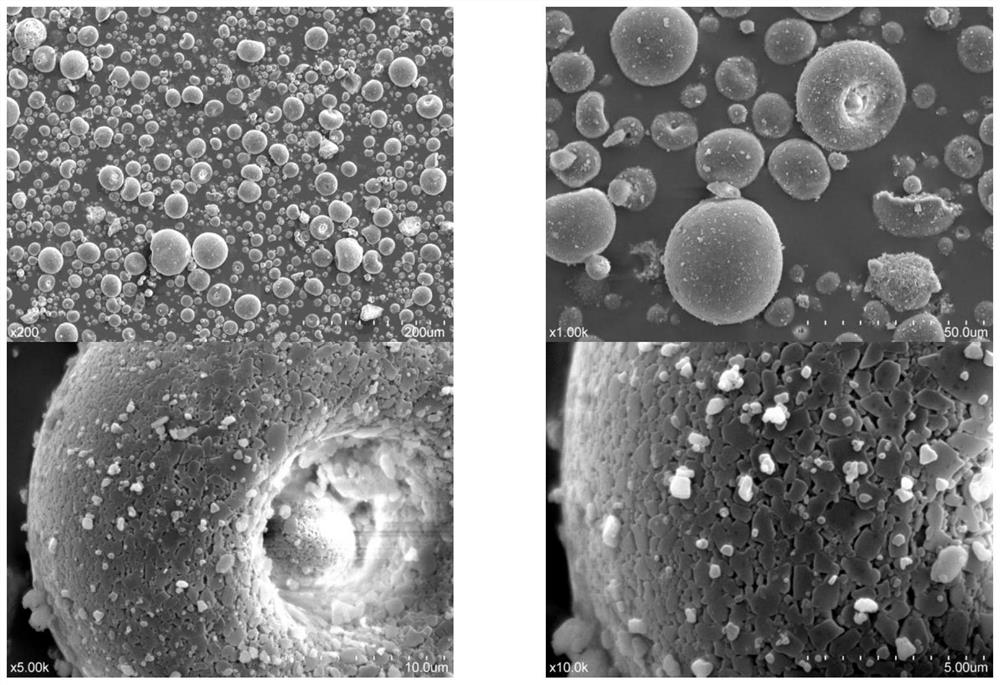
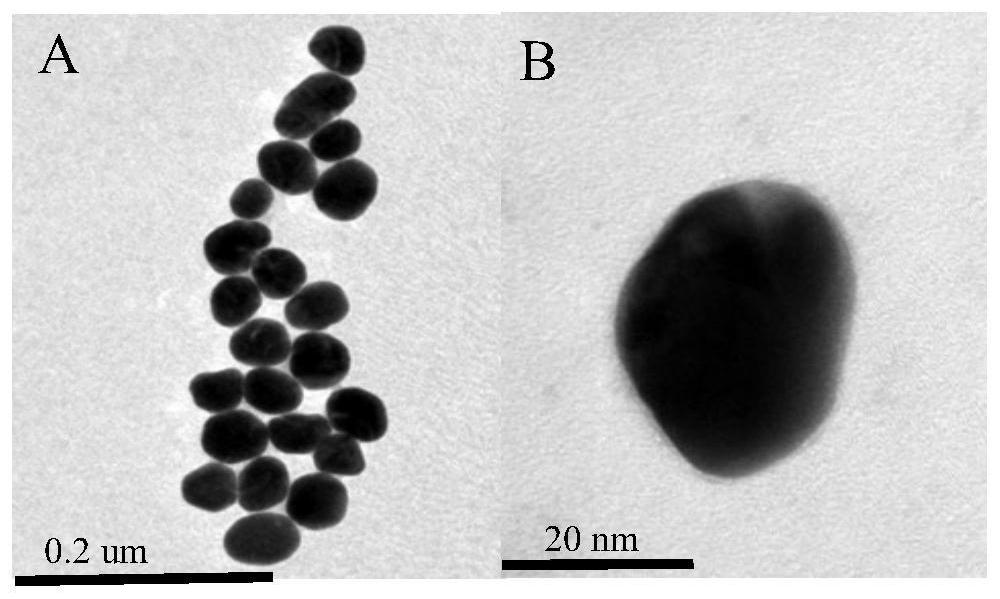
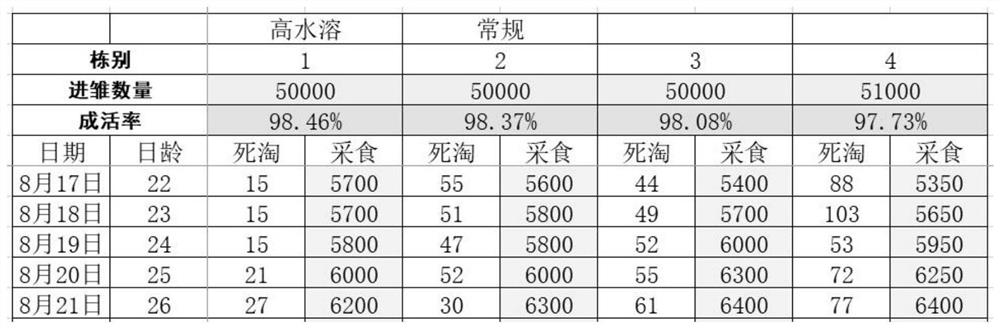
Patents
Literature
41 results about "Chlortetracycline Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A tetracycline with broad-spectrum antibacterial and antiprotozoal activity. Chlortetracycline hydrochloride is bacteriostatic and inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit, thereby preventing the addition of amino acids to the growing peptide chain. This tetracycline is active against a wide range of gram-positive and gram-negative organisms, spirochetes, rickettsial species, certain protozoa and Mycoplasma and Chlamydia organisms.
Chlortetracycline premix and preparation method thereof
ActiveCN103284956AAvoid joiningImprove complianceAntibacterial agentsTetracycline active ingredientsAdhesiveChlortetracycline Hydrochloride
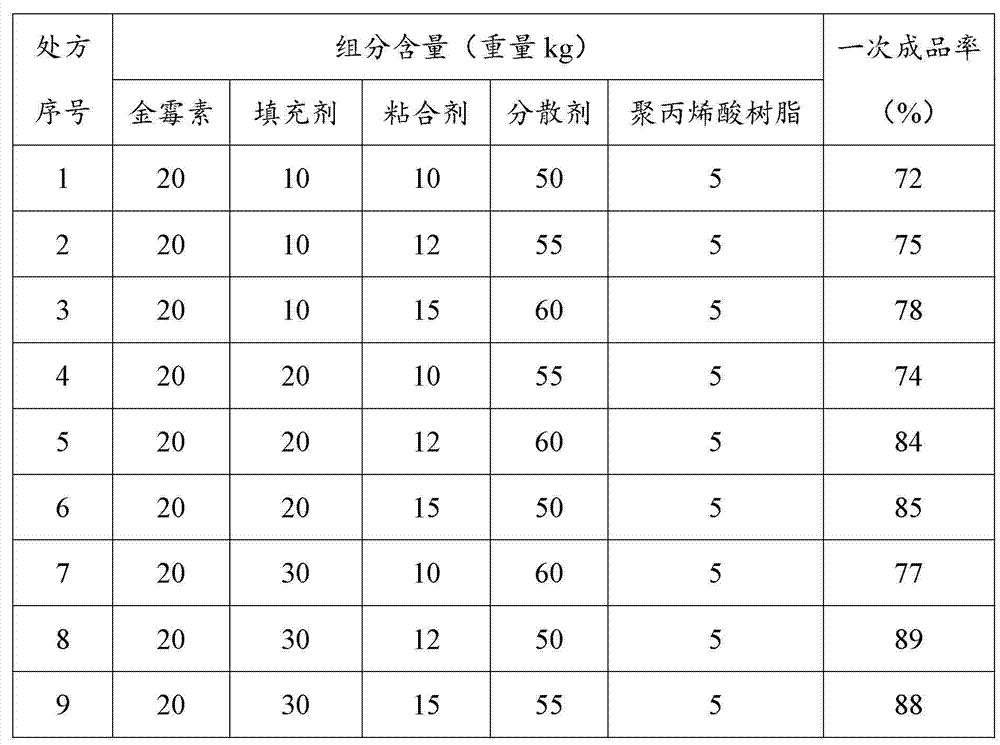
The invention provides a chlortetracycline premix and a preparation method thereof. By weight, the premix contains 10-20 parts of chlortetracycline hydrochloride, 50-60 parts of calcium carbonate, 10-30 parts of corn starch, 10-15 parts of an adhesive, and 5-10 parts of enteric polyacrylic resin, wherein the adhesive is microcrystalline cellulose and / or dextrin. The prescription of the chlortetracycline premix provided in the invention is more suitable for a dry granulation process, can significantly improve one-time yield and increase product fluidity and slow release property, thus solving the difficulties of a dry granulation process of the chlortetracycline premix. During mass production, the chlortetracycline hydrochloride premix can achieve good uniformity, and generates less dust. In addition, the premix can guarantee that the chlortetracycline hydrochloride is incompletely released in gastric juice, thus enhancing the utilization degree of chlortetracycline hydrochloride.
Owner:JINHE ANIMAL PHARMA
Formula of veterinary chlortetracycline hydrochloride soluble powder and preparation method thereof
InactiveCN104622811AIncrease absorption concentrationPrevent oxidation and discolorationAntibacterial agentsPowder deliveryChlortetracycline HydrochloridePharmacology
The invention discloses a formula of veterinary chlortetracycline hydrochloride soluble powder and a preparation method thereof. The soluble powder is prepared from the following components in percentage by weight: 10-50 percent of chlortetracycline hydrochloride, 0.2-3.0 percent of sodium dodecyl sulfate, 0.1-1.0 percent of ethylenediamine tetraacetic acid disodium salt and the balance of glucosum anhydricum. The chlortetracycline hydrochloride is utilized as a raw material, sodium dodecyl sulfate is added as an absorption enhancer, and ethylenediamine tetraacetic acid disodium salt is utilized as an antioxidant and a chelating agent, so that the stability and high efficiency of chlortetracycline hydrochloride soluble powder are ensured.
Owner:PIZHOU ZHENGKANG BIOTECH
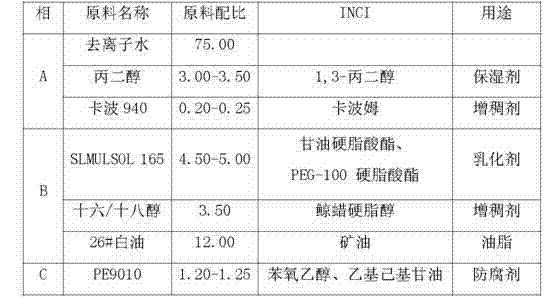
Method for preparing standard substance for detecting antibiotics in cosmetics
ActiveCN101904806AAccurate detectionCosmetic preparationsToilet preparationsCosmetic creamChlortetracycline Hydrochloride
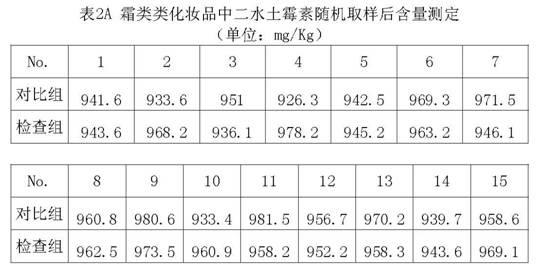
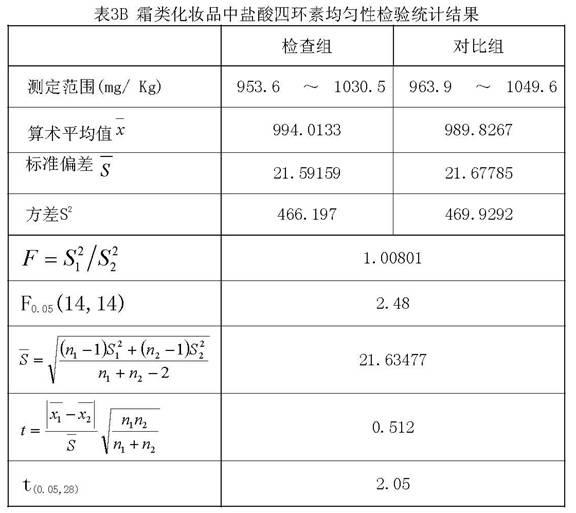
The invention relates to a method for preparing a standard substance for detecting antibiotics in cosmetics. The standard substance is formed by mixing aqueous solution of the antibiotics or solution of propylene glycol and cosmetic cream, wherein the cosmetic cream comprises the following components in percentage by weight: 73 to 78 percent of de-ionized water, 3 to 3.5 percent of propylene glycol and 0.2 to 0.25 percent of carbomer in phase A, 4.5 to 5 percent of stearate, 3 to 4 percent of cetyl alcohol and stearyl alcohol and 10 to 14 percent of 26# white oil in phase B and 1.2 to 1.25 percent of phenoxyethanol and ethylhexylglycerin in phase C; and the concentration of the antibiotics in the standard substance reaches 0.1g / 100mL. The method has the advantages that: the antibiotics and the cosmetic cream are mixed to form the standard substance for detecting the antibiotics such as minocycline hydrochloride, oxytetracycline dehydrate, tetracycline hydrochloride, chlortetracycline hydrochloride, doxycycline hydrochloride and chloramphenicol in the cosmetic; and the standard substance is the same as a sample of the cosmetic containing the antibiotics in the actual use; thus the detection of the antibiotics in the cosmetic conforms to the state of the actual application better and is more accurate.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH +1
Stable and efficient chlortetracycline hydrochloride soluble powder for livestock and preparation technology thereof
InactiveCN104721143AStable and efficient preparation processReduce interferencePowder deliveryTetracycline active ingredientsAnimal scienceChlortetracycline Hydrochloride
The invention discloses stable and efficient chlortetracycline hydrochloride soluble powder for livestock and a preparation technology thereof. The powder mainly comprises the following raw materials in percentage by weight: 10-15 percent of chlortetracycline hydrochloride, 0.2-3.0 percent of lauryl sodium sulfate, 0.1-1.0 percent of ethylenediamine tetraacetic acid disodium and anhydrous dextrose in balancing amount. The raw materials are crushed and screened respectively, and then are fully and uniformly mixed by an equivalent incremental method. According to the powder, chlortetracycline hydrochloride is adopted as a raw material, lauryl sodium sulfate is added as an absorption promoter, and ethylenediamine tetraacetic acid disodium salt is added as an antioxidant and a chelating agent, so that the stability and high efficiency of the chlortetracycline hydrochloride soluble powder are guaranteed.
Owner:CP UNITED ANIMAL PHARMA TECH JIANGSU
Chlortetracycline hydrochloride soluble powder and preparation method thereof
InactiveCN110507616AImprove solubilityIncrease dissolution rateAntibacterial agentsPowder deliveryChlortetracycline hclSolubility
The invention discloses chlortetracycline hydrochloride soluble powder and a preparation method thereof. The chlortetracycline hydrochloride soluble powder comprises the following raw materials (by mass): 10-20% of chlortetracycline hydrochloride, 10-40% of urea, 0-20% of a drying agent, 5-10% of a stabilizer and the balance of a carrier. By adding urea into the raw materials of the chlortetracycline hydrochloride soluble powder, solubility of the chlortetracycline hydrochloride soluble powder in water can be improved; dissolvability and dissolution rate of the chlortetracycline hydrochloridesoluble powder in water are improved; the stirring and dissolving time of the chlortetracycline hydrochloride soluble powder in use is greatly shortened; moreover, the chlortetracycline hydrochloridesoluble powder can be stably stored after being dissolved in water with different hardness, precipitation is avoided, the content of chlortetracycline hydrochloride in the solution is stable, the lossof the chlortetracycline hydrochloride soluble powder in use is reduced, and a drinking line is prevented from being blocked by precipitates. The chlortetracycline hydrochloride soluble powder is suitable for the situation that the water quality difference of farms in different regions is large.
Owner:FOSHAN STANDARD BIO TECH
Method for preparing chlortetracycline hydrochloride
InactiveCN105624222AReduce pollutionImprove securityMicroorganism based processesCarboxylic acid amide separation/purificationChlortetracycline HydrochlorideBULK ACTIVE INGREDIENT
The invention discloses a method for preparing chlortetracycline hydrochloride and belongs to the field of medicine synthesis. The method aims to solve the problems that at present, a second solvent crystallization method is mostly adopted for producing chlortetracycline hydrochloride in China, the secondary crystallization process is long, much solvent waste liquid is generated by production, and product quality and active ingredients are low. According to the method, strains in aureomycin fungus residues are extracted for culture, then a modified additive solution is utilized to conduct screening modification on cultured strains, meanwhile fermentation is conducted under the acidic condition, the content of aureomycin in the fermentation process is raised so that the aureomycin can be acidized easily, finally adsorption is conducted through attapulgite, the ultrasonic stripping is conducted, concentration and drying are conducted, and the chlortetracycline hydrochloride high in quality and activity is obtained.
Owner:CHANGZHOU LANXU CHEM CO LTD
Aureomycin micro-capsule premix and preparation method thereof
ActiveCN105832701AProlong the effective timeReduce the effect of absorptionAntibacterial agentsTetracycline active ingredientsSucrosePolyethylene terephthalate glycol
The invention belongs to the field of feed additives, and particularly relates to an aureomycin micro-capsule premix and a preparation method thereof. The premix is prepared from, by weight, 15-30 parts of chlortetracycline hydrochloride, 10-24 parts of sucrose ester, 40-60 parts of a capsule wall polymer, 4-8 parts of xanthan gum, 8-12 parts of polyvinylpyrrolidone and 50-70 parts of water, wherein sucrose ester includes 5-12 parts of sucrose ester with the HLB value of 3-5 and 5-12 parts of sucrose ester with the HLB value of 12-14, and the capsule wall polymer is prepared from a poly(lactide-glycolide acid) copolymer and polyvinyl alcohol acetic acid polyethylene terephthalate at the weight ratio of (0.5-2):(2-4). Aureomycin micro-capsules are prepared with a W / O / W duplicate-latices method, the obtained aureomycin micro-capsules are high in drug loading capacity and have good light and heat stability and slow release performance, aureomycin can be prevented from being dissolved out or damaged in the stomach and can be slowly released after entering the intestinal tract, and thus the drug function is improved.
Owner:GUANGZHOU CARDLO BIOCHEM TECH
Veterinary-use intestinal targeted antibacterial pellet and preparation method thereof
ActiveCN102600184AReduce first pass effectImprove bioavailabilityAntibacterial agentsTetracycline active ingredientsFormularyChlortetracycline Hydrochloride
The invention discloses a veterinary-use intestinal targeted antibacterial pellet. The veterinary-use intestinal targeted antibacterial pellet comprises a main drug and auxiliary drugs, wherein the main drug is composed of valnemulin hydrochloride and chlortetracycline hydrochloride; the mass of the valnemulin hydrochloride is 1-5% of that of the pellet; the mass of the chlortetracycline hydrochloride is 5-16 times of that of the valnemulin hydrochloride; the pellet sequentially comprises a valnemulin hydrochloride core, an intestinal targeted coating layer, a chlortetracycline hydrochloride drug layer and an outer coating layer from inside to outside. The invention further discloses a preparation method of the veterinary-use intestinal targeted antibacterial pellet. Through the combination of the method and the formula, the valnemulin hydrochloride and the chlortetracycline hydrochloride are adopted for preparing the pellet; the pellet is released in a gradient form in an animal digestion system; the chlortetracycline hydrochloride is firstly released and absorbed by the stomach and the intestine; the valnemulin hydrochloride is rapidly released and absorbed by the intestinal tract with the pH value larger than or equal to 6.8, so that an excellent intestinal tract sterilization effect is achieved, the drug absorbed by the colon can be prevented from suffering from the first-pass effect of the liver and the intestine, the haemoconcentration and bioavailability of the valnemulin hydrochloride can be improved, and an excellent treatment effect for systemic diseases is obtained.
Owner:山东胜利生物工程有限公司
Chlortetracycline hydrochloride soluble powder and preparation method thereof
ActiveCN107951843AImprove stabilityImprove solubilityAntibacterial agentsPowder deliveryGlycineTreatment effect
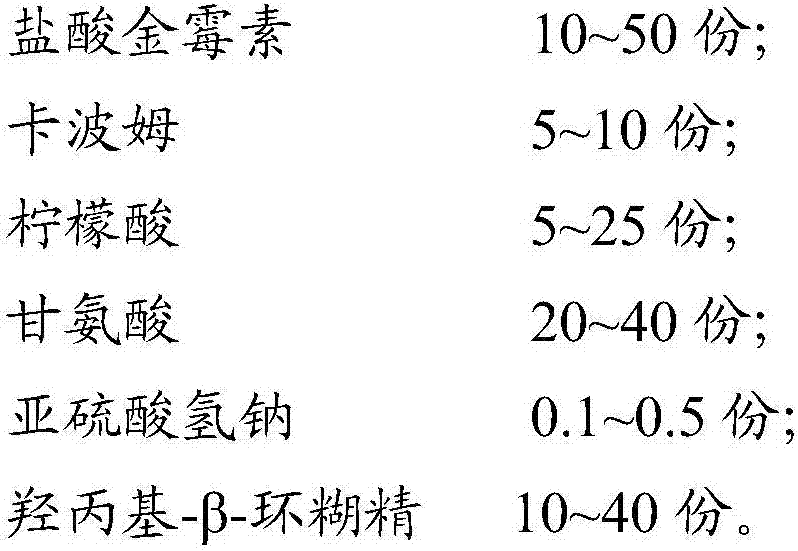
The invention provides a chlortetracycline hydrochloride soluble powder and belongs to the technical field of veterinary drugs. The chlortetracycline hydrochloride soluble powder is prepared from, bymass, 10-50 parts of chlortetracycline hydrochloride, 5-10 parts of carbomer, 5-25 parts of citric acid, 20-40 parts of glycine, 0.1-0.5 part of sodium hydrogen sulfite and 10-40 parts of hydroxypropyl-beta-cyclodextrin. The invention also provides a preparation method of the chlortetracycline hydrochloride soluble powder. The preparation method includes the steps of weighing the above raw materials by mass, and respectively crushing the raw materials; mixing the crushed raw materials with water and shaking to obtain raw material aqueous solution; drying the raw material aqueous solution andgranulating to obtain the chlortetracycline hydrochloride soluble powder. The chlortetracycline hydrochloride soluble powder prepared by the method has good stability and good solubility, and treatment effects are obviously better than that of the traditional chlortetracycline hydrochloride soluble powder.
Owner:PUCHENG CHIA TAI BIOCHEM
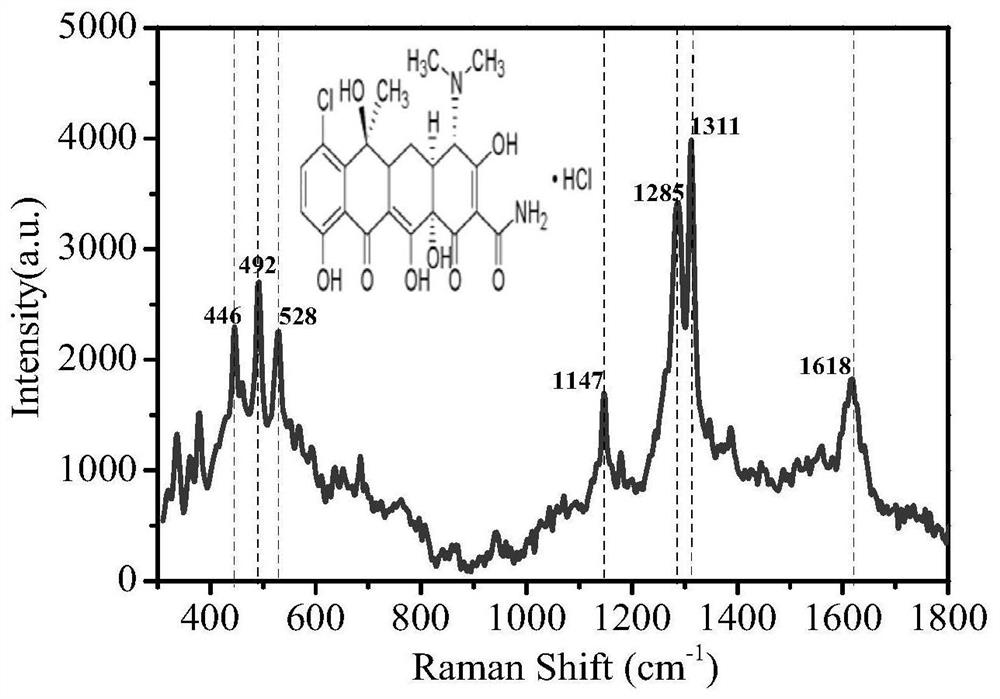
Surface Enhanced Raman Spectroscopy (SERS) detection method for chlortetracycline hydrochloride
ActiveCN109540869ATo achieve the purpose of concentration and enrichmentHigh spike recoveryRaman scatteringChlortetracycline HydrochlorideSurface-enhanced Raman spectroscopy
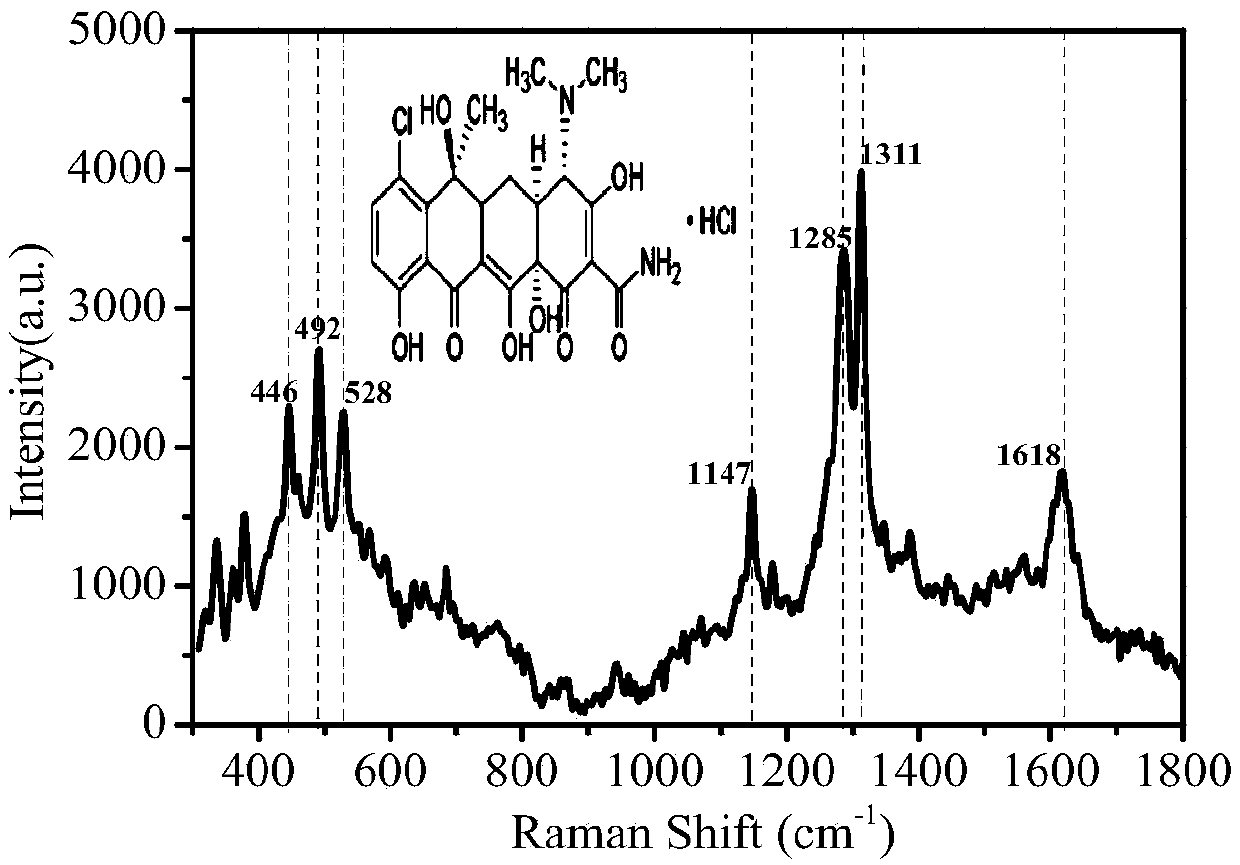
The invention relates to a Surface Enhanced Raman Spectroscopy (SERS) detection method for chlortetracycline hydrochloride and relates to detection of tetracycline antibiotics. Au nanoparticle sol isprepared by reducing chloroauric acid with sodium citrate, and gold nanoparticles with uniform particle size are prepared by controlling the concentration of reactants, the reaction time, the reactiontemperature and the stirring speed; a SERS substrate is prepared with gold sol under constant temperature conditions. the pH of a chlortetracycline hydrochloride solution is controlled, fluorescent quencher is added, surface enhanced Raman detection is carried out on chlortetracycline hydrochloride on the SERS substrate, with the increase of the concentration of chlortetracycline hydrochloride, aRaman peak of chlortetracycline hydrochloride at a specific wavelength is gradually enhanced, and the intensity of the Raman characteristic peak of chlortetracycline hydrochloride is proportional tothe amount of chlortetracycline hydrochloride so as to carry out quantitative analysis and detection on chlortetracycline hydrochloride. The method has the advantages of low detection limit, high sensitivity, good reproducibility of detection, fast detection speed, high standard recovery rate of samples and the like and can meet the requirement for rapid analysis and detection.
Owner:JIMEI UNIV
Oral ulcer ointment
InactiveCN109453111ASignificant effectReduce the smellTetracycline active ingredientsAerosol deliveryParaffin waxMedicine
The invention discloses oral ulcer ointment, which is prepared from the following components in parts by weight: 18-25 parts of chlortetracycline hydrochloride, 13-16 parts of vaseline and 3-6 parts of liquid paraffin; a preparation method of the oral ulcer ointment comprises the following steps: S1, carrying out pretreatment: sterilizing the liquid paraffin and the vaseline at a high temperature,and then cooling to the room temperature for later use; S2, adding the chlortetracycline hydrochloride into a mortar, adding the liquid paraffin and mixing into paste; then, adding the vaseline in 2-4 times, and evenly grinding to obtain the oral ulcer ointment. The oral ulcer ointment provided by the invention can rapidly diminish inflammation and relieve pain, is odorless, and is simple in rawmaterials and low in cost.
Owner:李萍
Preparation for treating periodontosis
ActiveCN103536680ARelieve painEasy to operateInorganic boron active ingredientsHydroxy compound active ingredientsVitamin CVitamin B12
The invention provides a preparation for treating periodontosis. The preparation comprises 0.001-3wt% of an iodine preparation, 0.1-0.2wt% of a bactericide, 0.5-1wt% of nystatin, 0.25-0.5wt% of chlortetracycline hydrochloride, 0.05-0.15wt% of a steride anti-inflammatory agent, 0.05-0.1wt% of vitamin C, 0.01-0.03wt% of vitamin B1, 0.001-0.01wt% of vitamin B12, 0.2-1wt% of glycerin, 0.01-0.3wt% of one of indigo naturalis, calculus bovis factitius, felwort, amur corktree bark, borneol or menthol, and balance distilled water. The preparation has the advantages of simple operation, small patient pain, good compliance, no operation treatment, low cost, short treatment course, good treatment effects, and simple and convenient doctor operation.
Owner:李文军
Formula and preparation method of veterinary injectant for preventing and treating endometritis
InactiveCN104188987ABroad spectrum antibacterialImprove antibacterial propertiesAntibacterial agentsTetracycline active ingredientsPathogenic microorganismSide effect
The invention discloses a formula of veterinary injectant for preventing and treating endometritis. The injectant prepared according to the formula of every 1000mL of veterinary injectant for preventing and treating the endometritis comprises the following components by weight: 50-200g of chlortetracycline hydrochloride, 0.1-0.2g of cosolvent, 1-2g of antioxidant, 100-800g of composite organic solvent and 1-150g of pH value regulator. The invention also discloses a preparation method of the veterinary injectant for preventing and treating the endometritis. The veterinary injectant for preventing and treating the endometritis has the characteristics of having broad antibacterial spectrum, targeting, strong function, quick response and lasting effect and being directly accessed to lesions, can prevent and inhibit reproduction of pathogenic microorganisms in uterus and can avoid side effect due to excessive use of antibiotics.
Owner:PIZHOU ZHENGKANG BIOTECH
Preparation method of hydrochloric acid aureomycin
ActiveCN103833591AHigh extraction yieldSolve pollutionCarboxylic acid amide separation/purificationChlortetracycline HydrochlorideNanofiltration
The invention relates to a preparation method of hydrochloric acid aureomycin; the technical steps are listed below: firstly acidifying aureomycin broth; adjusting pH to 2-2.5; standing; continuously adjusting pH to 4-4.5; standing; adding water to reduce viscosity to 3000-4000cps pretreatment; carrying out membrane filtration, nanofiltration, crystallization, washing, salt forming, leaching and drying to obtain hydrochloric acid aureomycin powders. The method can improve extraction yield of the hydrochloric acid aureomycin, reduces production cycle and cost, and improves hydrochloric acid aureomycin output.
Owner:宁夏泰瑞制药股份有限公司
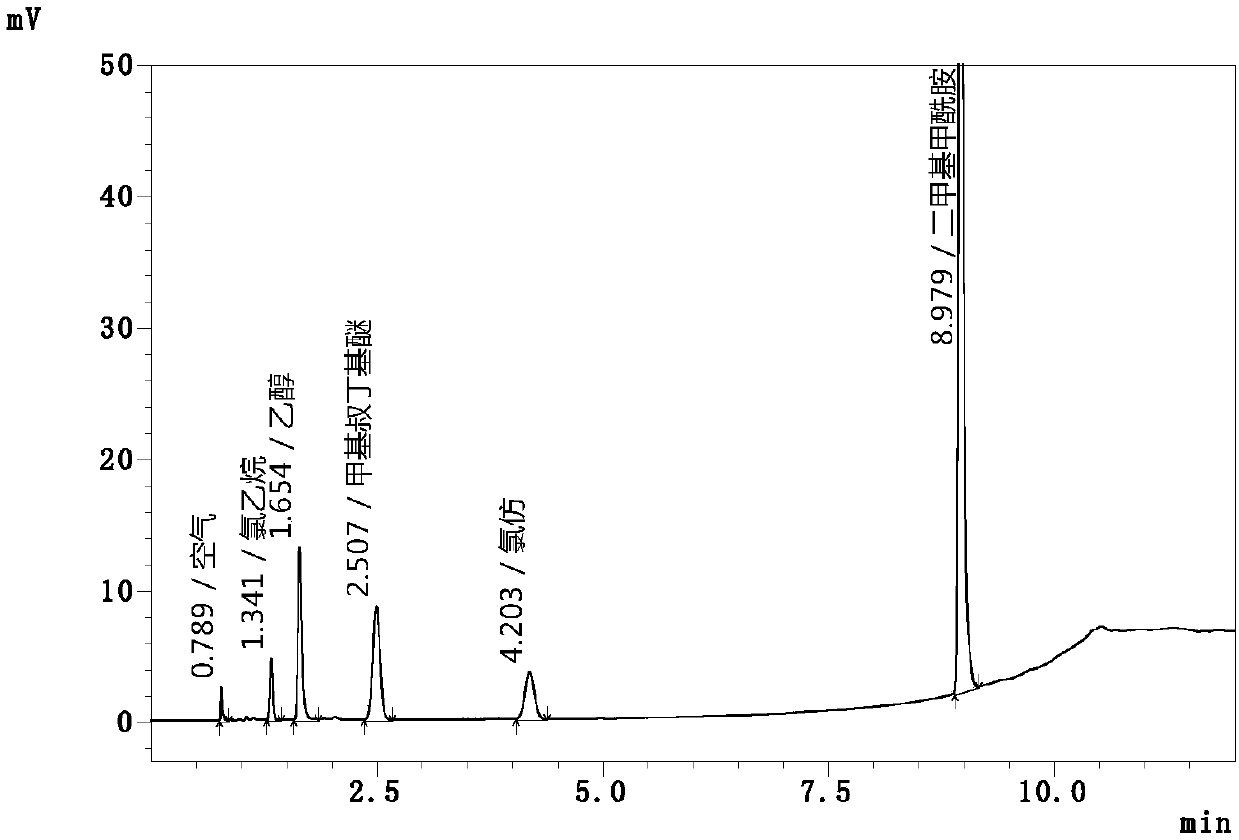
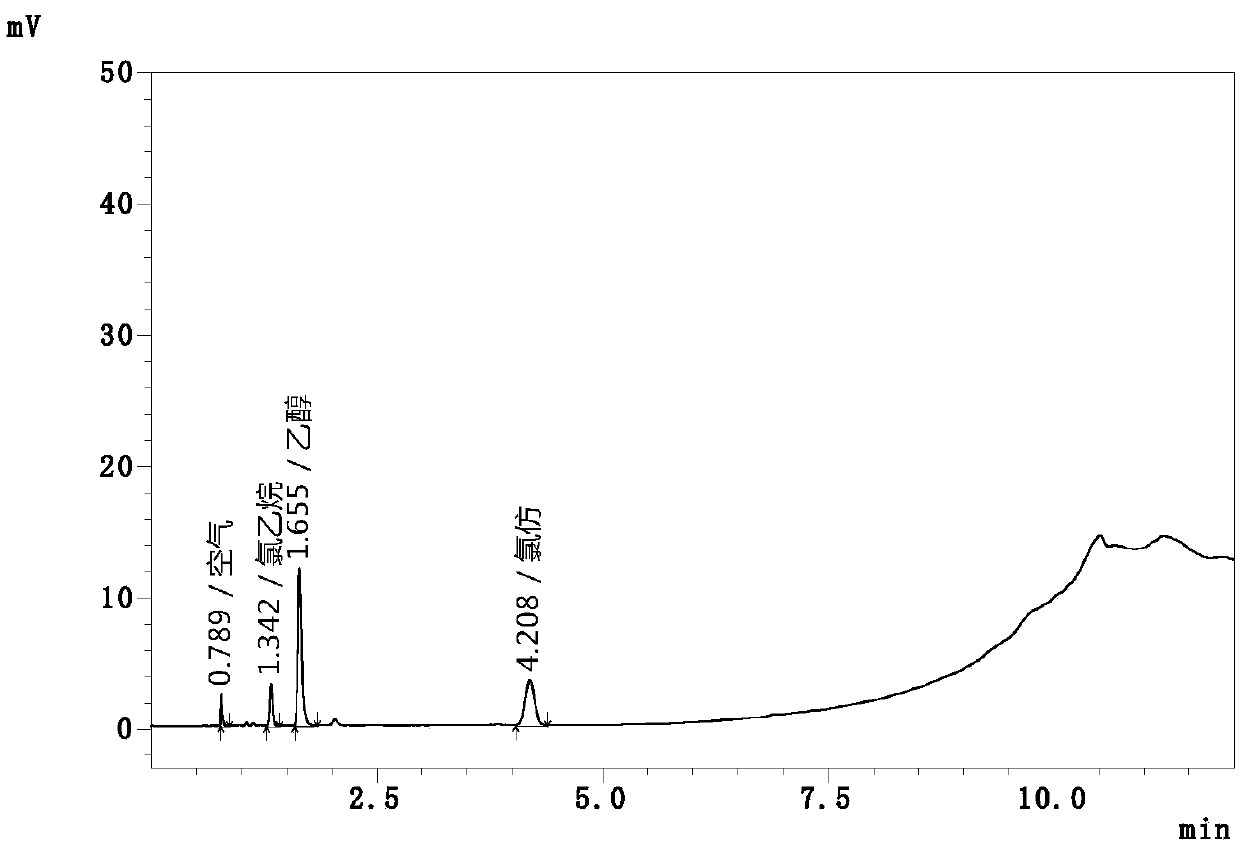
Automatic headspace sampling gas chromatography method for determining chloroethane and chloroform residual in chlortetracycline hydrochloride
InactiveCN107727764AAchieving Simultaneous DetectionIncrease temperatureComponent separationTest efficiencyAir velocity
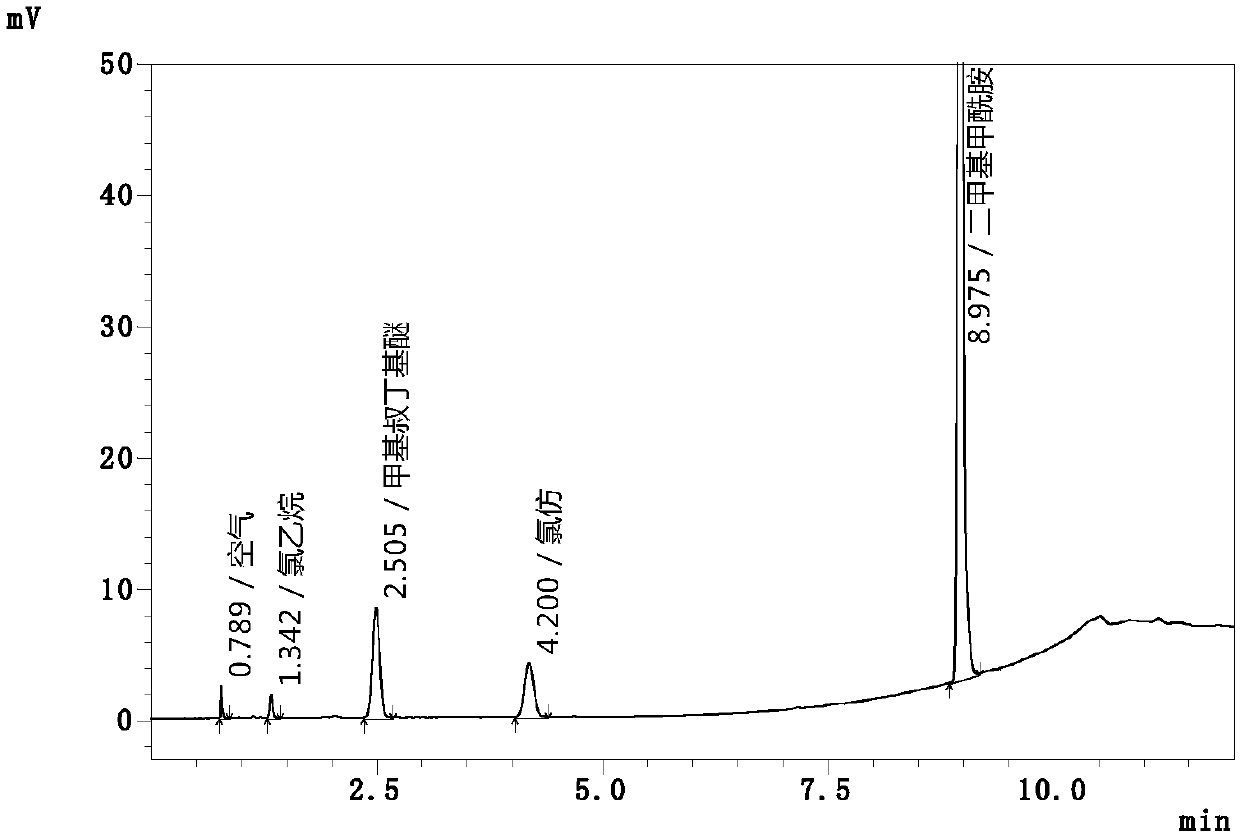
The invention relates to an automatic headspace sampling gas chromatography method for determining chloroethane and chloroform residual in chlortetracycline hydrochloride and belongs to the technicalfield of analysis detection. The method has gas chromatography conditions of a sampling temperature of 25 DEG C, a column temperature of 40 DEG C, nitrogen gas as carrier gas, a carrier gas flow of 60mL / min, a detector temperature of 250 DEG C, a split ratio of 5: 1, a sampling volume of 1.0mL, air velocity of 250mL / min and operation time of 12min, and headspace sampling parameters of a constant temperature oven temperature of 60 DEG C, sample constant temperature time of 30min, sample pressing time of 2.0min, pressing balance time of 0.1min, leading time of 0.1min, sampling time of 1.0min anda sampling amount of 1mL. The method utilizes automatic headspace sampling, optimizes a sampling temperature and carrier gas flow, provides a test precision and sensitivity, shortens the existing detection time 26min to 12min, improves test efficiency, saves carrier gas, realizes simultaneous detection of chloroform and chloroethane, further improves test efficiency and reduces a detection cost.
Owner:金河生物科技股份有限公司
Preparation method of ultrahigh water-soluble chlortetracycline hydrochloride
PendingCN114276267ASmall particle sizeIncrease the areaOrganic compound preparationCarboxylic acid amide separation/purificationChlortetracycline HydrochlorideDouble salt
The invention discloses a preparation method of ultrahigh water-soluble chlortetracycline hydrochloride. The preparation method specifically comprises the following steps: 1) acidifying fermentation liquor; 2) double salt; 3) coarse crystallization; 4) extracting; (5) recrystallizing; (6) performing high-pressure homogenization: performing high-pressure homogenization treatment on the material obtained in the step (1) by taking purified water as a dispersion medium according to a water-material ratio of 1: (1-1.5) for 10-12 times; and 7) spray drying: carrying out spray drying treatment on the material obtained in the previous step, controlling the feeding frequency to be 20Hz and the air inlet temperature to be 175-180 DEG C to obtain the ultrahigh water-soluble chlortetracycline hydrochloride.
Owner:PUCHENG CHIA TAI BIOCHEM
A kind of sers detection method of aureomycin hydrochloride
ActiveCN109540869BTo achieve the purpose of concentration and enrichmentHigh spike recoveryRaman scatteringChlortetracycline HydrochlorideNanoparti cles
A SERS detection method for aureomycin hydrochloride relates to the detection of tetracycline antibiotics. Au nanoparticle sol is prepared by reducing chloroauric acid with sodium citrate, and gold nanoparticles with uniform particle size are obtained by controlling the concentration of reactants, reaction time, reaction temperature and stirring speed; SERS substrate, control the pH of aureomycin hydrochloride solution, add a fluorescence quencher, and perform surface-increased Raman detection on aureomycin hydrochloride on the SERS substrate, as the concentration of aureomycin hydrochloride increases, The Raman peak of chlortetracycline hydrochloride at a specific wavelength is gradually enhanced, and the intensity of the Raman characteristic peak of chlortetracycline hydrochloride is proportional to the amount of chlortetracycline hydrochloride. Quantitative analysis detection. It has the advantages of low detection limit, high sensitivity, good detection reproducibility, fast detection speed, high recovery rate of sample addition, etc., and can meet the requirements of rapid analysis and detection.
Owner:JIMEI UNIV
Composition for treating porcine respiratory tract infection and preparation method of composition
InactiveCN107441111AReduce chance of drug resistanceImprove antibacterial propertiesTetracycline active ingredientsAntiinfectivesChlortetracycline HydrochlorideFumaric acid
The invention relates to a composition for treating porcine respiratory tract infection and a preparation method of the composition. The composition is prepared from the following raw materials in percentage by weight: 1-5 % of marbofloxacin, 1-5 % of tiamulin fumarate, 5-15 % of tilmicosin phosphate, 5-15 % of lincomycin hydrochloride, 5-15 % of chlortetracycline hydrochloride, the balance of anhydrous glucose. All the components are grinded, the obtained powder is screened by adopting a No.5 sieve, the screened powder is sufficiently and uniformly mixed, and thus the composition for treating porcine respiratory tract infection is obtained. Compared with the prior art, the composition has the advantages that the plurality of medicines which can be mixed for use are comprehensively selected, the situation that some pathogenic bacteria have drug resistance to some medicines, consequently, treatment failure is caused, is avoided, the drug resistance probability of pathogens is reduced, so that the pathogenic microorganism resisting range is wide, the antibacterial effect is strong, and disease recovery is facilitated.
Owner:JIANGXI AOXIN BIOTECH CO LTD +1
Film for treating oral ulcer and preparation method thereof
ActiveCN100596280CGood flexibilityHigh tensile strengthHydroxy compound active ingredientsTetracycline active ingredientsChlortetracycline HydrochlorideOral ulcers
The invention discloses a plastering film for treating ulcerative stomatitis and its preparation, wherein the medicament is prepared from honeysuckle flower, menthol, vitamin B2, boneol, tetracaine hydrochloride, sorbic acid, prednisone acetate, chlortetracycline hydrochloride, glycerin, stevioside, ethanol, polyvinyl alcohol PVA17-88 and purified water.
Owner:GUIZHOU BAIQIANG PHARMA
Veterinary chlortetracycline premix and preparation method thereof
ActiveCN105997894AReduce releaseExtended absorption timeAntibacterial agentsTetracycline active ingredientsSucroseChlortetracycline Hydrochloride
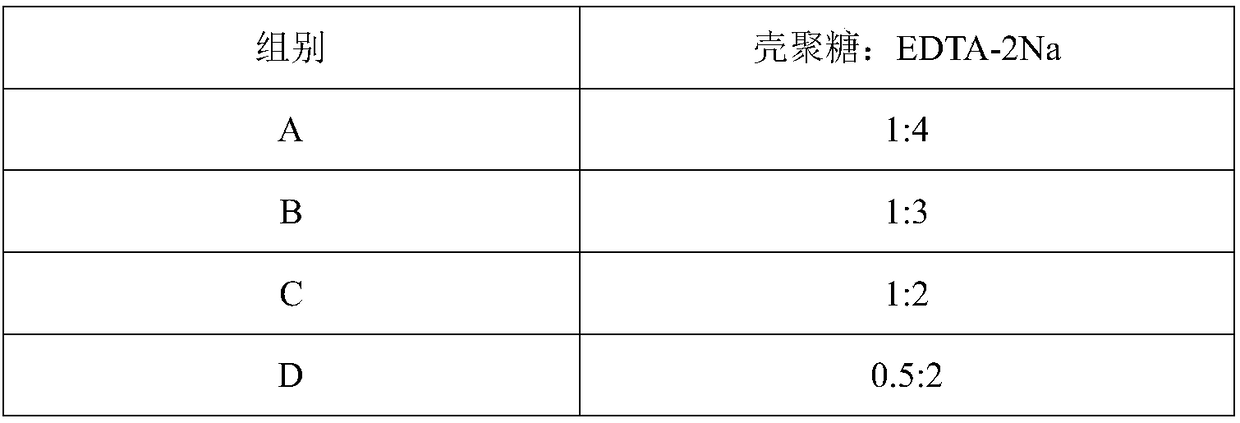
The invention belongs to the field of feed additives and in particular relates to a veterinary chlortetracycline premix and a preparation method thereof. The premix is prepared from the following raw materials in parts by weight: 20 to 30 parts of chlortetracycline hydrochloride, 30 to 40 parts of N-trimethyl chitosan, 20 to 40 parts of starch, 2 to 6 parts of polyvinylpyrrolidone K30, 1 to 3 parts of a chitosan-EDTA (Ethylene Diamine Tetraacetic Acid) complex and 10 to 18 parts of water, and further comprises 4 to 8 parts of a sweetener; the sweetener is one of sucrose, lactose, sorbitol and mannitol. The premix is prepared into grains by adopting a wet-process granulation process and the prepared premix grains are uniform and have high granulation rate and stable quality. Furthermore, the premix can be used for effectively reducing complex compounds formed by the chlortetracycline hydrochloride and metal ions in gastrointestinal tracts; the chlortetracycline hydrochloride can be incompletely released in gastric juice and the bioavailability of the chlortetracycline hydrochloride is improved. The preparation process of the premix provided by the invention is simple and industrial application is easy to realize.
Owner:GUANGZHOU CARDLO BIOCHEM TECH
Water-soluble aureomycin succinic acid monoester salt and preparation method thereof
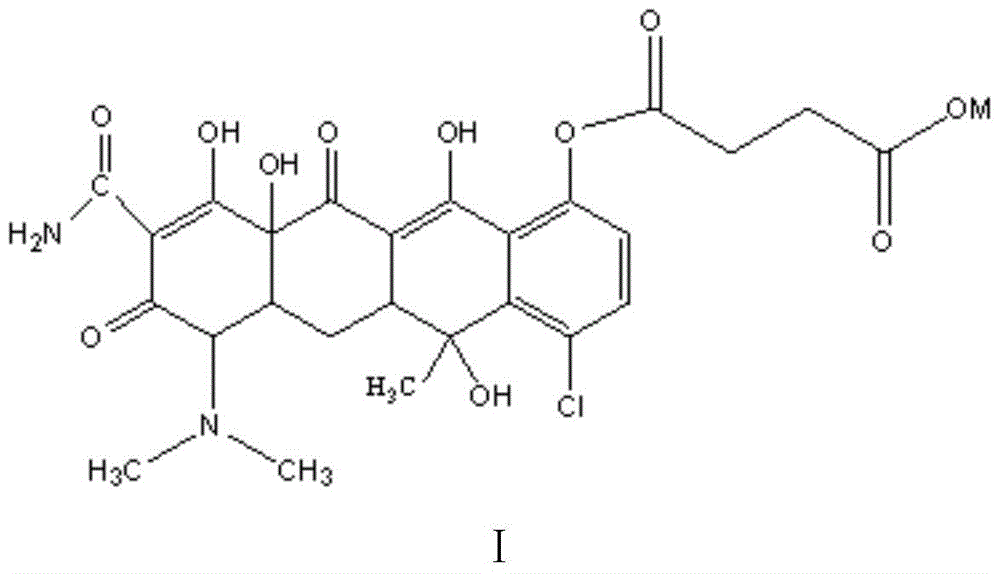
ActiveCN105001112BGrowth-promoting effectImprove immunityAntibacterial agentsOrganic compound preparationChemical synthesisArginine
The invention belongs to the technical field of chemical synthesis, and more specifically relates to a water soluble chlorotetracycline succinic acid monoester salt, and a preparation method thereof. The preparation method is used for modifying chlorotetracycline. The chlorotetracycline succinic acid monoester salt possesses a structure represented by formula I, wherein M is used for representing Na, K, meglumine, arginine, or lysine. According to the preparation method, grafting modification of chlorotetracycline with succinic anhydride is carried out so as to obtain the chlorotetracycline succinic acid monoester salt with water solubility better than that of chlorotetracycline, and broad-spectrum antibacterial property of the water soluble chlorotetracycline succinic acid monoester salt is the same as that of chlorotetracycline in animal bodies.
Owner:PUCHENG CHIA TAI BIOCHEM
HPLC Gradient Elution Method for Determination of Related Substances of Chlortetracycline Hydrochloride
ActiveCN111426772BDecrease the speed of increaseOvercome Retention Time DriftComponent separationChlortetracycline HydrochloridePhysical chemistry
The invention relates to a method for determining related substances of aureomycin hydrochloride by an HPLC gradient elution method. Specifically, the method for the present invention's determination of related substances in aureomycin hydrochloride or its preparations comprises the steps: (1) high performance liquid chromatography and chromatographic column are provided; (2) mobile phase is prepared; (3) test solution is prepared (4) Inject various test solutions into the liquid chromatograph respectively, measure according to the specified chromatographic conditions, record the chromatogram and calculate the content of each related substance in the test substance. The method of the present invention exhibits one or more excellent effects as described in the specification, such as low column pressure, small chromatographic peak drift and / or stable test solution.
Owner:江西省药品检验检测研究院
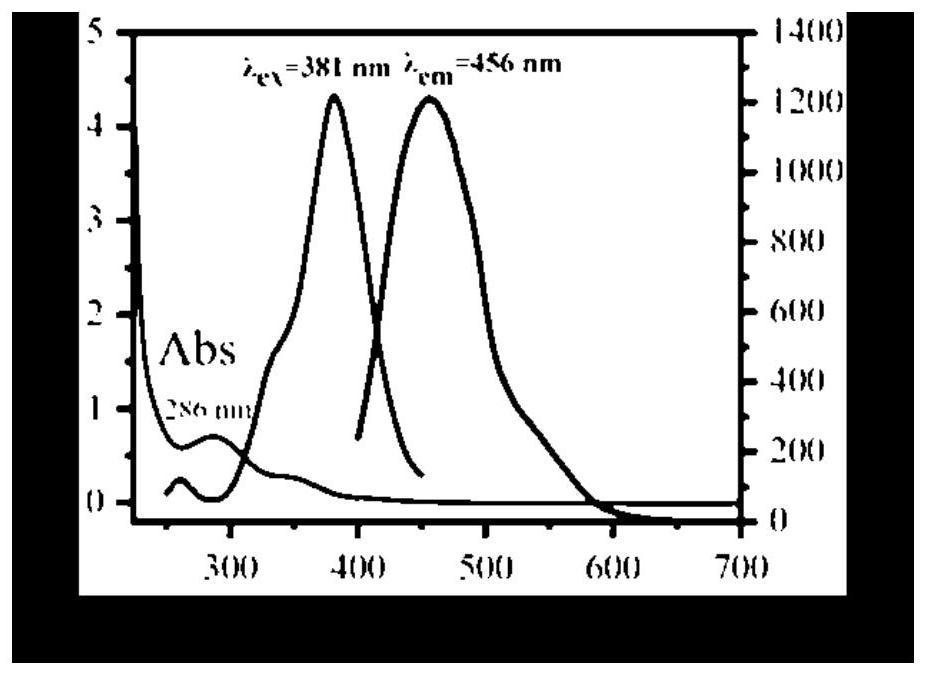
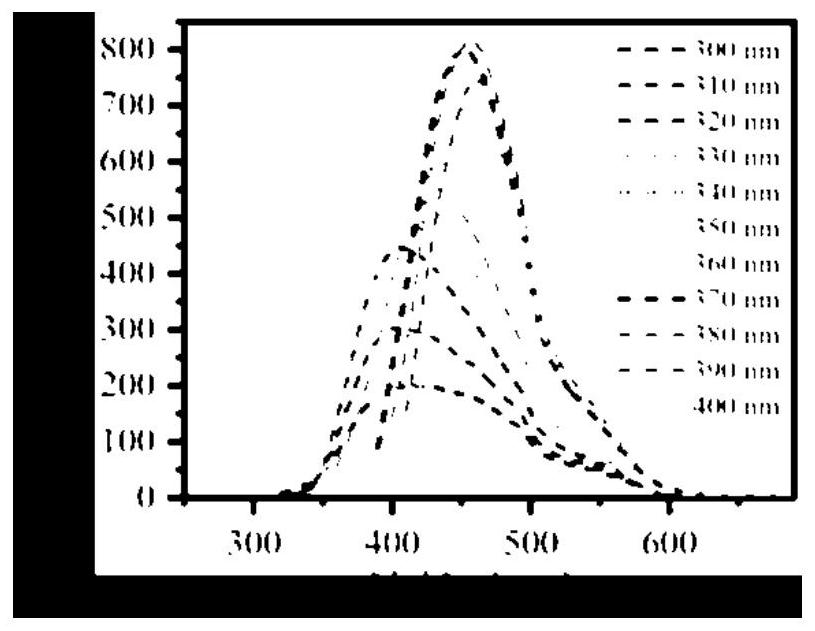
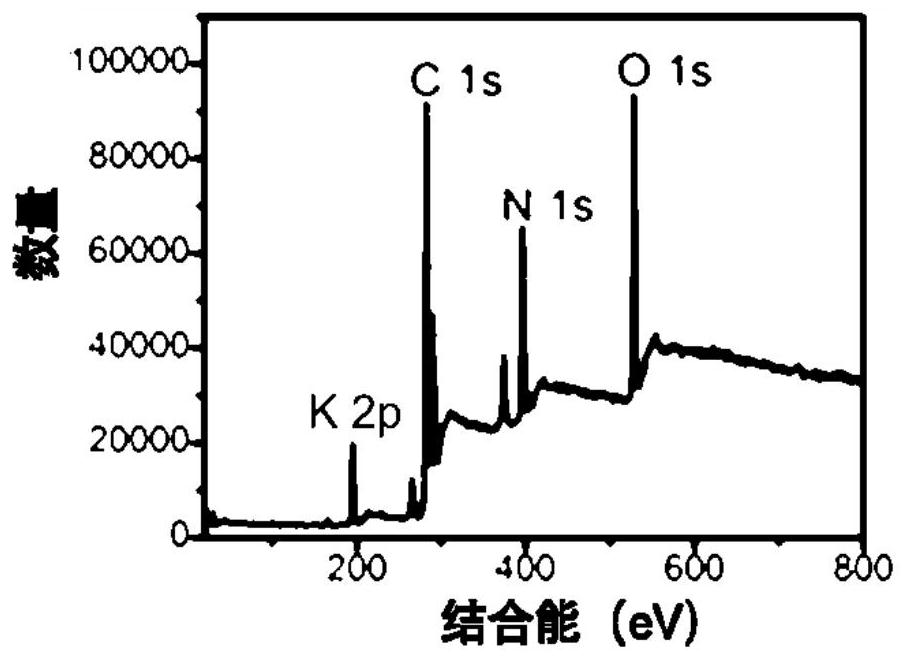
A potassium-nitrogen co-doped carbon dot based on steamer peel and its preparation method and application
ActiveCN113549450BWide variety of sourcesSave energyMaterial nanotechnologyFluorescence/phosphorescenceChlortetracycline HydrochlorideFluorescent quenching
The invention provides a potassium-nitrogen co-doped carbon dot based on steamer peel and its preparation method and application. In the invention, the potassium-nitrogen co-doped carbon dots are prepared by one-step hydrothermal reaction by taking steamer peel and ethylenediamine as precursors and secondary water as a solvent. The method for preparing carbon quantum dots in the invention is simple, low in cost and easy to obtain raw materials. The potassium-nitrogen co-doped carbon dots were synthesized from discarded steamer peels, which can realize the rational utilization of biomass waste, which not only saves energy, but also is beneficial to environmental protection. The prepared carbon quantum dots have excellent optical properties, and different detection mechanisms based on fluorescence enhancement, fluorescence ratio and fluorescence quenching can realize the identification and selective detection of tetracycline antibiotics. The carbon quantum dot of the invention can be used to detect the contents of aureomycin hydrochloride, tetracycline and oxytetracycline in milk, honey, fish and pork.
Owner:SHANXI UNIV
Preparation method of hydrochloric acid aureomycin
ActiveCN103833591BHigh extraction yieldSolve pollutionCarboxylic acid amide separation/purificationChlortetracycline HydrochlorideNanofiltration
Owner:宁夏泰瑞制药股份有限公司
Method for preparing standard substance for detecting antibiotics in cosmetics
ActiveCN101904806BAccurate detectionCosmetic preparationsToilet preparationsCosmetic creamChlortetracycline Hydrochloride
The invention relates to a method for preparing a standard substance for detecting antibiotics in cosmetics. The standard substance is formed by mixing aqueous solution of the antibiotics or solution of propylene glycol and cosmetic cream, wherein the cosmetic cream comprises the following components in percentage by weight: 73 to 78 percent of de-ionized water, 3 to 3.5 percent of propylene glycol and 0.2 to 0.25 percent of carbomer in phase A, 4.5 to 5 percent of stearate, 3 to 4 percent of cetyl alcohol and stearyl alcohol and 10 to 14 percent of 26# white oil in phase B and 1.2 to 1.25 percent of phenoxyethanol and ethylhexylglycerin in phase C; and the concentration of the antibiotics in the standard substance reaches 0.1g / 100ml. The method has the advantages that: the antibiotics and the cosmetic cream are mixed to form the standard substance for detecting the antibiotics such as minocycline hydrochloride, oxytetracycline dehydrate, tetracycline hydrochloride, chlortetracycline hydrochloride, doxycycline hydrochloride and chloramphenicol in the cosmetic; and the standard substance is the same as a sample of the cosmetic containing the antibiotics in the actual use; thus the detection of the antibiotics in the cosmetic conforms to the state of the actual application better and is more accurate.
Owner:SHANGHAI INST OF MEASUREMENT & TESTING TECH +1
Chlortetracycline premix
InactiveCN107714708AReduce releaseQuality improvementTetracycline active ingredientsDigestive systemChlortetracycline HydrochlorideN-trimethyl chitosan
The invention discloses a chlortetracycline premix, which consists of the following components in parts by weight: 15-25 parts of chlortetracycline hydrochloride, 10-20 parts of corn starch, 1-3 partsof N-trimethyl chitosan, 3-6 parts of a sweetening agent and 6-12 parts of a binding agent. The chlortetracycline premix, which is prepared in accordance with the prescription, is stable in quality and high in utilization rate; chlortetracycline, which is less released in gastric juice, can reach an intestinal tracts well so as to achieve good efficacy; and the chlortetracycline premix has a goodapplication prospect and is worthy of popularizing.
Owner:江苏昕宇药业有限公司
Enteric coating aureomycin premix
InactiveCN107811992AReasonable prescriptionPromote dissolutionTetracycline active ingredientsDigestive systemCelluloseChlortetracycline Hydrochloride
The invention discloses an enteric coating aureomycin premix, which comprises drug-loading pellets and a coating which is arranged outside the drug-loading pellets. The drug-loading pellets contain the following ingredients (by weight): 20-30 parts of chlortetracycline hydrochloride, 15-25 parts of low-substituted hydroxypropy cellulose, 5-10 parts of polyvinylpyrrolidone K30 and 1-3 parts of a sweetener. The coating contains the following ingredients (by weight): 20-30 parts of polyacrylic resin II, 2-6 parts of a plasticizer and 1-4 parts of talcum powder. According to the enteric coating aureomycin premix, polyacrylic resin II is insoluble in gastric juice but only soluble in intestinal juice such that aureomycin is effectively protected and won't be released or absorbed until reachingthe intestinal tract; the formula of the drug-loading pellets is reasonable, and the particles are small after disintegration of the low-substituted hydroxypropy cellulose, which is greatly beneficialto dissolution of the drug; and the enteric coating aureomycin premix has a good application prospect and is worthy of promotion.
Owner:江苏昕宇药业有限公司
Recovery method of demeclocy cline hydrochloride crystal mother liquor
InactiveCN1226278CReduce pollutionAvoid wastingCarboxylic acid amide separation/purificationRecovery methodScavenger
The invention discloses a method for recovering a chlortetracycline hydrochloride crystal mother liquor. The method includes the following steps: a. natural sedimentation of the chlortetracycline hydrochloride crystallization mother liquor at low temperature, and filtering; b. adjusting the pH of the filtrate with an alkaline solution, adding a filter aid, and filtering; c. dissolving the filter cake with an oxalic acid solution , add a purifying agent and filter; d. perform solvent extraction with multi-carbon alcohol or a mixed solution of multi-carbon alcohol and acetate, and cool the extract; e. use urea double salt, wash, crystallize, and dry. The method for recovering the chlortetracycline hydrochloride crystal mother liquor provided by the invention can make the recovered chlortetracycline hydrochloride content reach more than 80%, the titer unit can reach more than 900u / ml, and the recovery yield can reach more than 50%. The method of the invention effectively avoids the waste caused by discarding the chlortetracycline crystal mother liquor in the production process of chlortetracycline hydrochloride, can save 5% of the production cost, and simultaneously reduces the environmental pollution in the production process.
Owner:NORTH CHINA PHARMA GROUP CORP
Chlortetracycline hydrochloride dry suspension as well as preparation method and application thereof
PendingCN114099441ASuitable for industrial productionContinuous productionAntibacterial agentsPowder deliveryChlortetracycline HydrochlorideClinical tests
The invention relates to a chlortetracycline hydrochloride dry suspension as well as a preparation method and application thereof, and the chlortetracycline hydrochloride dry suspension comprises the following raw materials in percentage by mass: 15-35% of chlortetracycline hydrochloride, 1-15% of a suspending aid, 1-5% of a pH value regulator, 0.1-1% of a flavoring agent and 44.9-82.9% of a filler. The method comprises the following steps: S1, crushing and sieving chlortetracycline hydrochloride; s2, sieving other auxiliary materials; s3, weighing the chlortetracycline hydrochloride, the suspending aid, the pH value adjusting agent and the flavoring agent according to the proportion, feeding the materials into a stirring tank in vacuum, and adding a proper amount of purified water to form a suspension; s4, carrying out spray drying on the suspension, and sieving with a 80-mesh sieve; and S5, uniformly mixing the product obtained by spraying with a filling agent in a mixing machine to obtain the chlortetracycline hydrochloride dry suspension. The product disclosed by the invention is acidic and can be used together with drugs which have synergistic interaction with chlortetracycline hydrochloride, the palatability of an aqueous solution is good, and clinical test results show that the product disclosed by the invention improves the clinical effect and the blood concentration of chlortetracycline hydrochloride.
Owner:金河牧星(重庆)生物科技有限公司
A kind of aureomycin premix for veterinary use and preparation method thereof
ActiveCN105997894BReduce releaseExtended absorption timeAntibacterial agentsTetracycline active ingredientsSucroseChlortetracycline Hydrochloride
The invention belongs to the field of feed additives and in particular relates to a veterinary chlortetracycline premix and a preparation method thereof. The premix is prepared from the following raw materials in parts by weight: 20 to 30 parts of chlortetracycline hydrochloride, 30 to 40 parts of N-trimethyl chitosan, 20 to 40 parts of starch, 2 to 6 parts of polyvinylpyrrolidone K30, 1 to 3 parts of a chitosan-EDTA (Ethylene Diamine Tetraacetic Acid) complex and 10 to 18 parts of water, and further comprises 4 to 8 parts of a sweetener; the sweetener is one of sucrose, lactose, sorbitol and mannitol. The premix is prepared into grains by adopting a wet-process granulation process and the prepared premix grains are uniform and have high granulation rate and stable quality. Furthermore, the premix can be used for effectively reducing complex compounds formed by the chlortetracycline hydrochloride and metal ions in gastrointestinal tracts; the chlortetracycline hydrochloride can be incompletely released in gastric juice and the bioavailability of the chlortetracycline hydrochloride is improved. The preparation process of the premix provided by the invention is simple and industrial application is easy to realize.
Owner:GUANGZHOU CARDLO BIOCHEM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com