Aureomycin micro-capsule premix and preparation method thereof
A premix, chlortetracycline technology, applied in the directions of microcapsules, capsule delivery, pharmaceutical formulations, etc., can solve the problems of prolonging the effective action time of chlortetracycline, unstable chlortetracycline, and reducing the number of administrations. Effective duration of action, reducing the number of doses, resolving unstable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
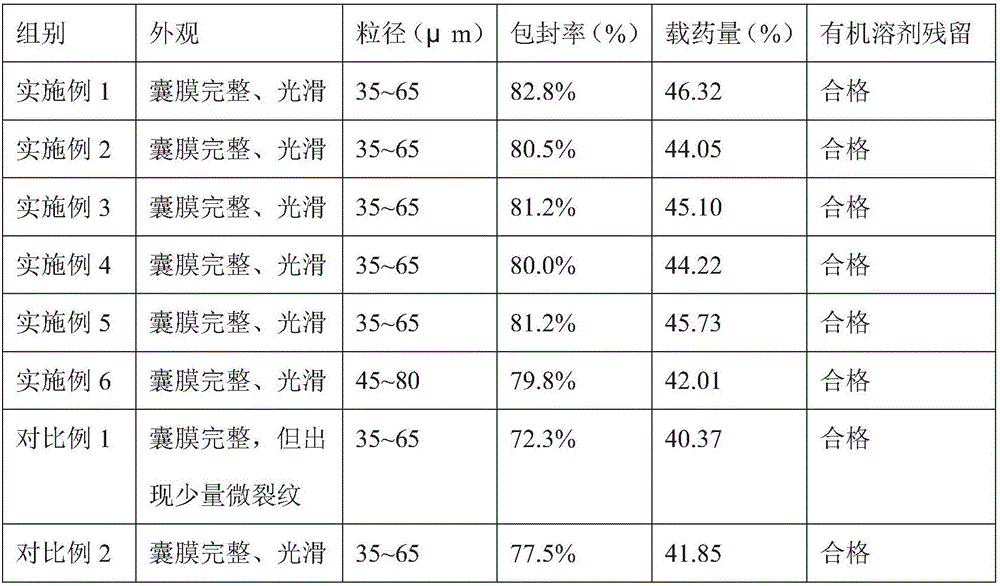
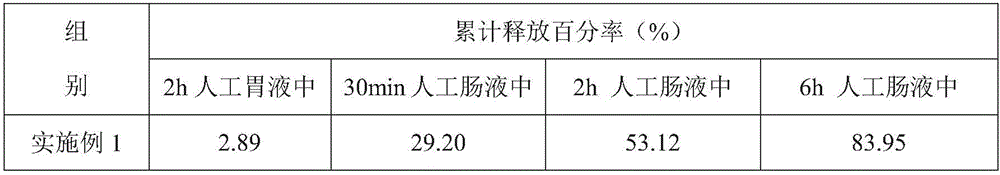
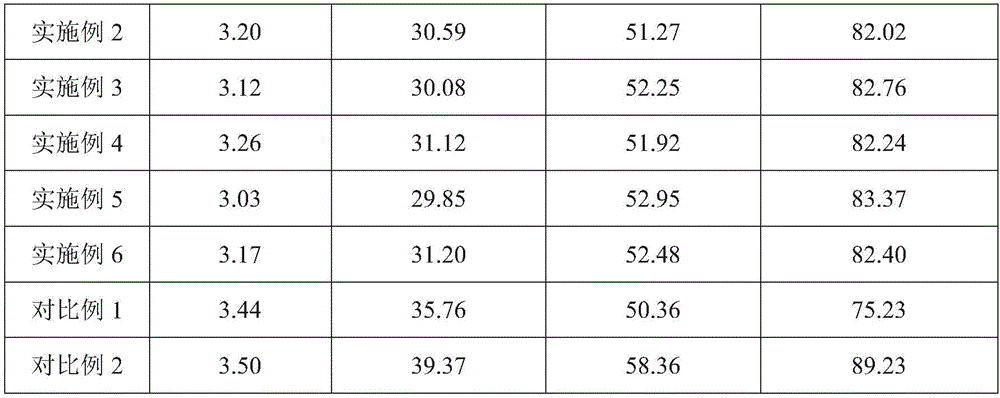
[0029] The aureomycin microcapsule premix of the embodiment of the present invention 1 comprises the raw material of following parts by weight:
[0030] 25 parts of aureomycin hydrochloride, 16 parts of sucrose ester, 50 parts of capsule wall polymer, 6 parts of xanthan gum, 10 parts of polyvinylpyrrolidone and 60 parts of water;
[0031] Wherein, the sucrose esters include 10 parts of sucrose esters with an HLB value of 4 and 6 parts of sucrose esters with an HLB value of 12; The ester is composed of a weight ratio of 1:2.5; the solvent of the capsule wall polymer is ethyl acetate, and the amount of ethyl acetate is 1.5 times the weight of the capsule wall polymer;
[0032] Preparation:
[0033] (1) Take the capsule wall polymer, add ethyl acetate and stir to dissolve, and add sucrose ester with an HLB value of 4, stir and disperse evenly to obtain an oil phase solution;
[0034] (2) Take aureomycin hydrochloride, add 1 / 3 amount of water, stir evenly, and then disperse in t...
experiment example 2
[0039] The chlortetracycline microcapsule premix of embodiment 2 of the present invention comprises the raw material of following parts by weight:
[0040] 15 parts of aureomycin hydrochloride, 10 parts of sucrose ester, 40 parts of capsule wall polymer, 4 parts of xanthan gum, 8 parts of polyvinylpyrrolidone and 50 parts of water;
[0041]Wherein, the sucrose esters include 6 parts of sucrose esters with an HLB value of 4 and 4 parts of sucrose esters with an HLB value of 12; The ester is composed of a weight ratio of 1:2.5; the solvent of the capsule wall polymer is ethyl acetate, and the amount of ethyl acetate is 1.5 times the weight of the capsule wall polymer;
[0042] The preparation method refers to Example 1.
experiment example 3
[0044] The aureomycin microcapsule premix of embodiment 3 of the present invention comprises the raw material of following parts by weight:
[0045] 30 parts of aureomycin hydrochloride, 24 parts of sucrose ester, 60 parts of capsule wall polymer, 8 parts of xanthan gum, 12 parts of polyvinylpyrrolidone and 70 parts of water;
[0046] Wherein, the sucrose esters include 14 parts of sucrose esters with an HLB value of 4 and 10 parts of sucrose esters with an HLB value of 12; The ester is composed of a weight ratio of 1:2.5; the solvent of the capsule wall polymer is ethyl acetate, and the amount of ethyl acetate is 1.5 times the weight of the capsule wall polymer;
[0047] The preparation method refers to Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com