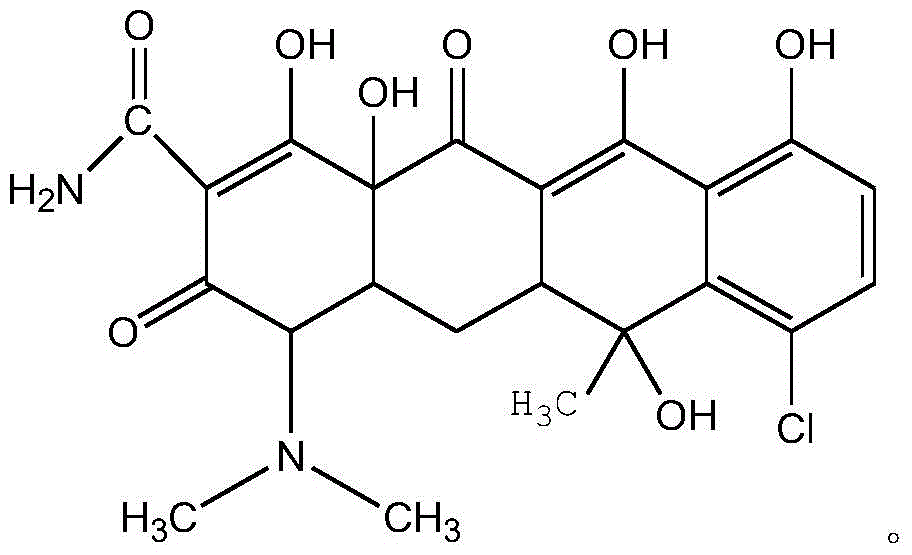
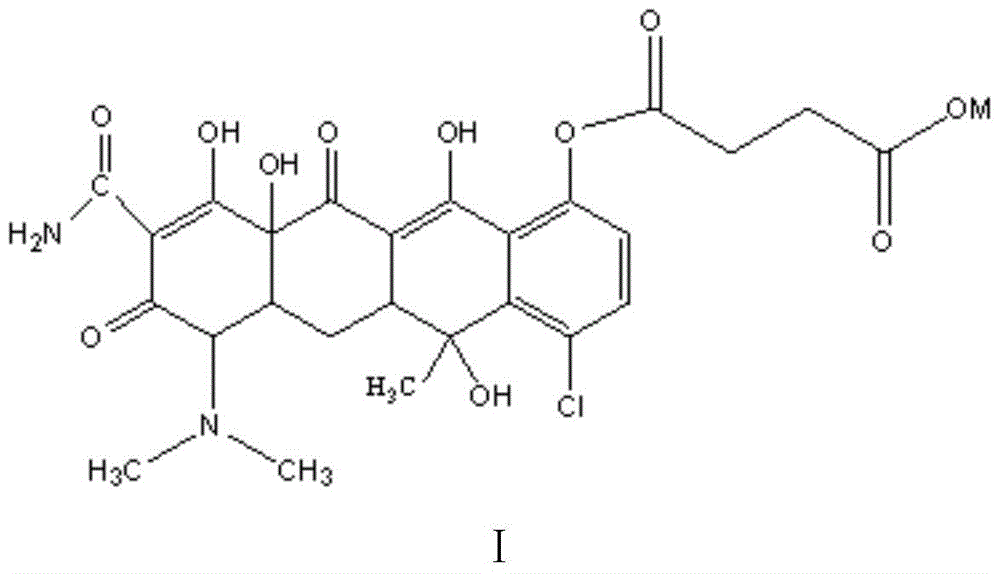
Water-soluble aureomycin succinic acid monoester salt and preparation method thereof
A technology of succinic acid monoester and chlortetracycline, applied in the field of water-soluble chlortetracycline succinic acid monoester salt and preparation thereof, can solve the problems of body irritation or pain, poor solubility, bad smell or taste, etc. The effect of promoting animal growth, good application prospects, and enhancing immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1, the preparation of aureomycin succinic acid monoester sodium salt
[0029] Dissolve chlortetracycline hydrochloride (1.0mol) in 40% ethanol aqueous solution, add sodium carbonate (1.2mol) solid and stir, and stir the solution at 80°C to form a homogeneous phase; add succinic anhydride (5.0mol) and bicyclic Hexylcarbodiimide (1.2mol) was stirred under reflux for 3 hours, acidified to pH=6 with hydrochloric acid, and cooled to obtain aureomycin succinic acid monoester as a solid.
[0030] Dissolve the obtained aureomycin succinate monoester (1mol) in ethanol, reflux, add sodium hydroxide (1.0mol) in batches, stir, and cool to generate the target product aureomycin succinate monoester sodium salt . The product is light yellow powder with a yield of 77.8%. As determined by HPLC, the purity of the product was 98.6%; as determined by FAB-MS (fast atom bombardment mass spectrometry), the molecular weight of the product was 600.9, which was consistent with the t...
Embodiment 2
[0031] Embodiment 2, the preparation of aureomycin succinic acid monoester sodium salt
[0032] Dissolve chlortetracycline hydrochloride (1.0mol) in 90% acetone aqueous solution, add sodium carbonate (1.5mol) solid stirring, stir the solution at 60 ℃ and become homogeneous; Add succinic anhydride (1.2mol) and 4- Dimethylaminopyridine (1.8 mol), stirred at reflux for 0.5 hour, acidified with hydrochloric acid to pH=6, cooled to obtain aureomycin succinic acid monoester solid.
[0033] Dissolve the obtained aureomycin succinate monoester (1mol) in isopropanol, reflux, add sodium hydroxide (2.0mol) in batches, stir, and cool to generate the target product aureomycin succinate monoester sodium salt. The product is light yellow powder with a yield of 81.3%. As determined by HPLC, the purity of the product was 98.58%; as determined by FAB-MS, the molecular weight of the product was 600.1, which was consistent with the theoretically calculated molecular weight of the target product...
Embodiment 3
[0034] Embodiment 3, the preparation of aureomycin succinate monoester potassium salt
[0035] Dissolve chlortetracycline hydrochloride (1.0mol) in 70% aqueous ethylene glycol solution, add sodium carbonate (1.0mol) solid and stir, and stir the solution at 30°C to become homogeneous; add succinic anhydride (3.0mol) and Triethylamine (4.5mol) was stirred under reflux for 5 hours, acidified with hydrochloric acid to pH=6, and cooled to obtain aureomycin succinic acid monoester as a solid.
[0036] The obtained aureomycin succinic acid monoester (1mol) was dissolved in N,N-dimethylformamide, refluxed, and potassium hydroxide (1.5mol) was added in batches, stirred, and cooled to generate the target product gold Potassium monomycin succinate. The product is light yellow powder with a yield of 85.9%. As determined by HPLC, the purity of the product was 99.1%; as determined by FAB-MS, the molecular weight of the product was 617.2, which was consistent with the theoretically calcula...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com