HPLC Gradient Elution Method for Determination of Related Substances of Chlortetracycline Hydrochloride
A technology of chlortetracycline hydrochloride and related substances, applied in the field of medicine, can solve the problems such as large retention time drift, high pressure of chromatography system, insufficient stability of test solution and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
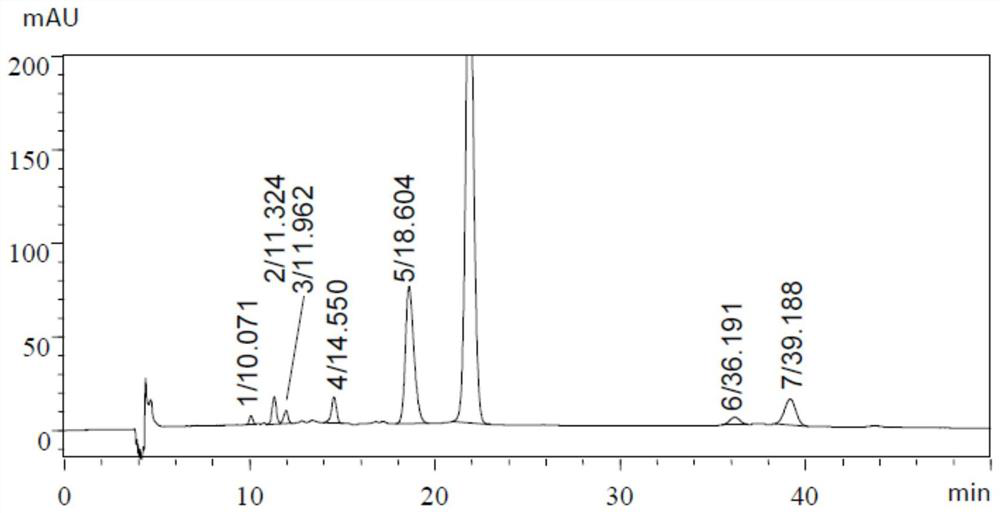
[0082] Embodiment 1: British Pharmacopoeia 2019 edition determines the method for aureomycin hydrochloride related substances
[0083] Solution preparation:
[0084] Test solution: dissolve 25.0 mg of test product with mobile phase B and dilute to 25.0 mL with this mobile phase;
[0085] Reference solution (a): Dissolve 25.0 mg of aureomycin hydrochloride reference substance with mobile phase B and dilute to 25.0 mL with this mobile phase;
[0086] Reference solution (b): Dilute 1.0mL of the test solution with mobile phase B to 100.0mL;
[0087] Reference solution (c): Dilute 1.0mL of reference solution (b) to 10.0mL with mobile phase B;
[0088] Reference solution (d): Dissolve 5 mg of aureomycin system suitability reference substance (which contains impurities A, B, D, E, G, H, J, K and L) with mobile phase B and dilute with this mobile phase to 5mL;
[0089] Reference solution (e): Dissolve 25.0 mg of aureomycin hydrochloride reference substance in mobile phase B and ...
Embodiment 2
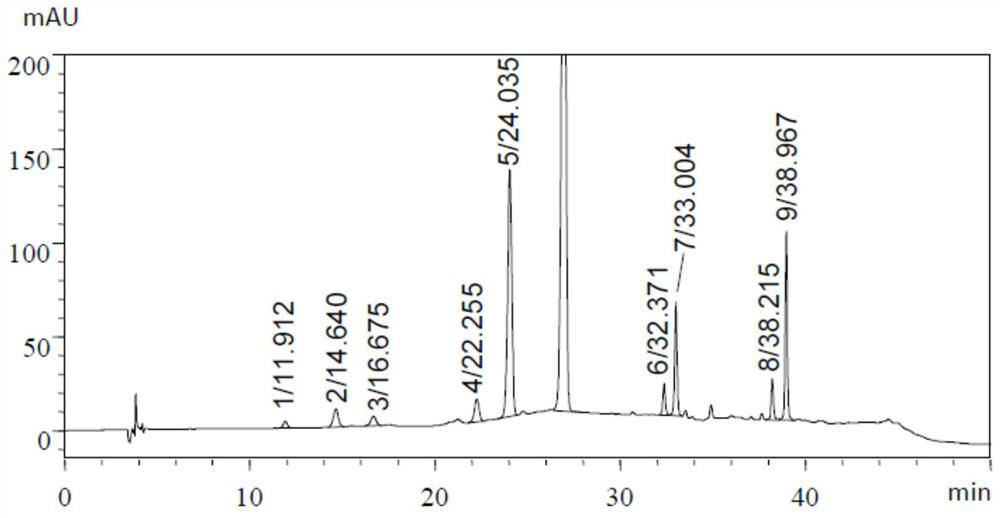
[0104] Embodiment 2: HPLC gradient elution method determines aureomycin hydrochloride related substances
[0105] The chromatographic conditions for isocratic elution used in the inspection of related substances in the chlortetracycline hydrochloride species recorded in the second part of the Chinese Pharmacopoeia in the 2015 edition are: octylsilane bonded silica gel (C8 column) as filler, high chlorine Acid-dimethyl sulfoxide-water (8:525:467) (pH<2.0) is the mobile phase, the column temperature is 45°C, and the detection wavelength is 280nm.
[0106] In this example, Ultimate XB-C8 (4.6×250mm, 5μm, chromatographic column 1) and InertSustainC8 (4.6×250mm, 5μm, chromatographic column 2) were used as chromatographic columns respectively, and the hydrochloric acid prepared in Example 1 was used according to the above-mentioned Chinese Pharmacopoeia method Chlortetracycline crude drug need testing solution is measured (the retention time of first pin aureomycin is about 21.8mi...
Embodiment 3
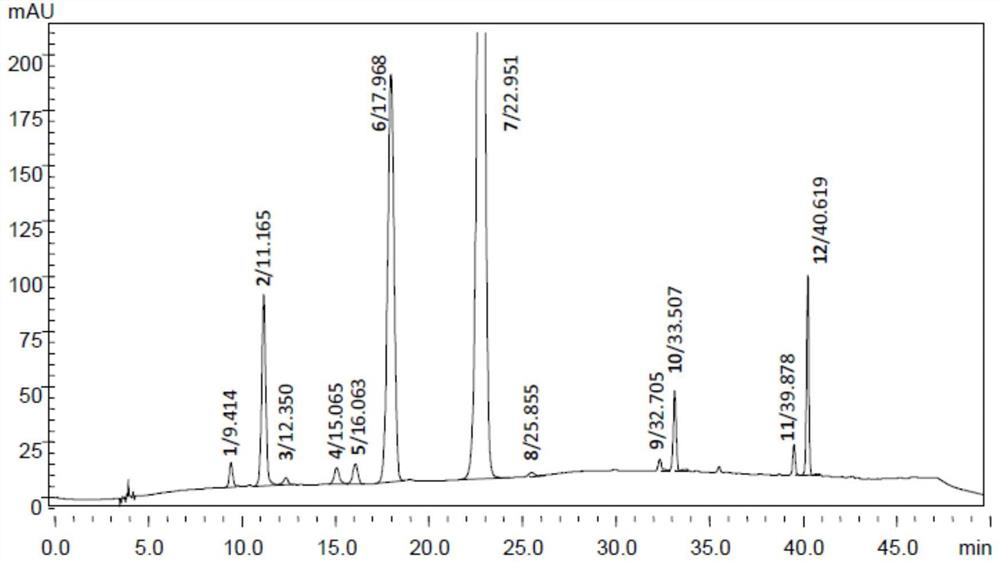
[0107] Embodiment 3: HPLC gradient elution method determines aureomycin hydrochloride related substances
[0108] The inventor has tried four kinds of mobile phase systems in the test: 1. ammonium acetate sodium perchlorate solution (pH 2.2)-methanol, 2. 0.05mol / L dipotassium hydrogen phosphate solution (triethylamine adjusts pH to 10.0)-methanol / acetonitrile, ③perchloric acid-water-methanol-acetonitrile, ④perchloric acid-dimethyl sulfoxide-water-acetonitrile-methanol. It was found that only dimethyl sulfoxide had a good modification effect on the peak shape symmetry of the chromatographic peak of aureomycin hydrochloride. Considering that both Chinese Pharmacopoeia (2015) and BP (2019) contain a relatively high proportion of dimethyl sulfoxide in the mobile phase, and the latter adopts ultra-high performance liquid chromatography, so referring to the gradient elution method of BP (2019), we will The ratio of perchloric acid and dimethyl sulfoxide in the mobile phase is g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com