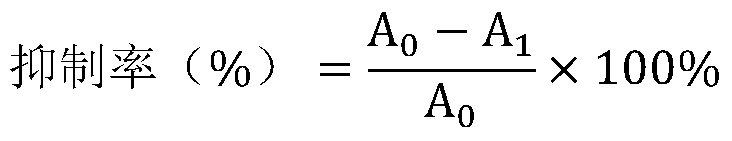Patents
Literature
54results about How to "Reduce medication burden" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
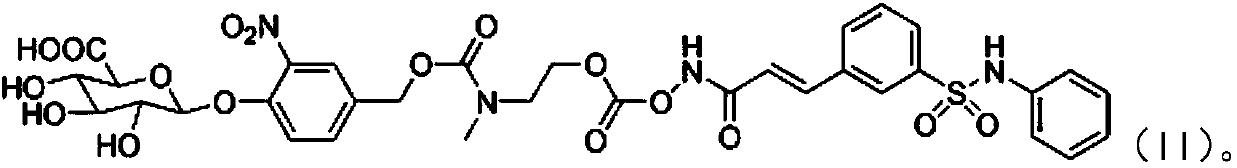
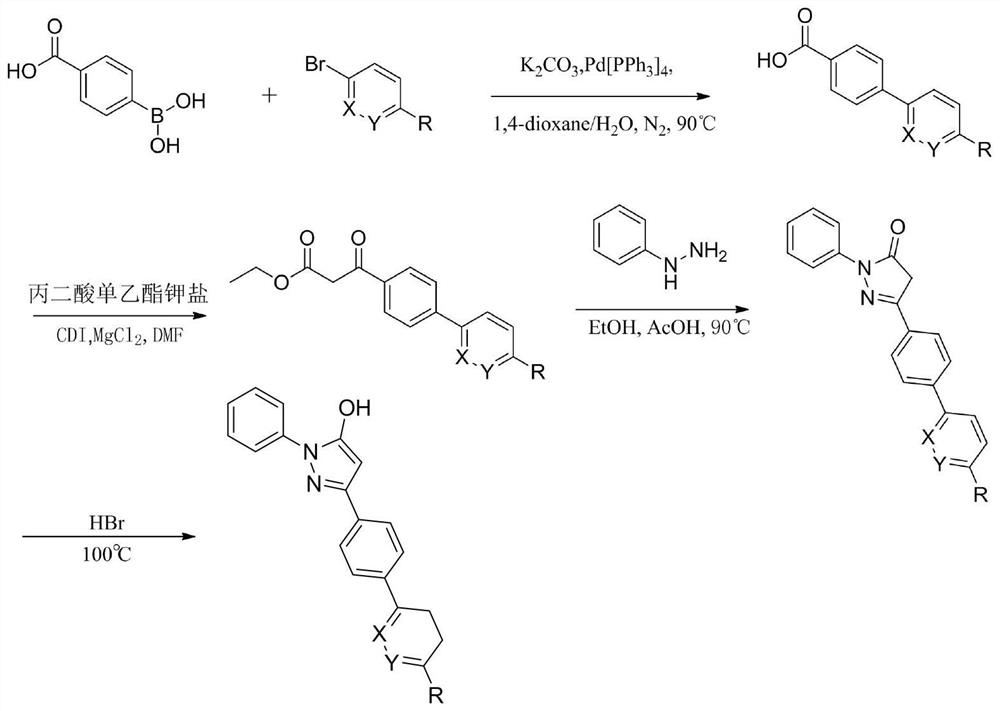
Synthesis process of adefovir dipivoxil raw medicine
ActiveCN101830932AReduce medication burdenSimple processGroup 5/15 element organic compounds2-ChloroethanolReagent
The invention provides an industrialized production and synthesis process of an adefovir dipivoxil raw medicine. The synthesis process comprises the following process routes of: reacting 2-chlorohydrin subjected to cholromethylation and triisopropyl phosphite to generate an adefovir lateral chain which is condensed and hydrolyzed with adenine to generate adefovir, and then carrying out condensation on the adefovir and chloromethyl pivalate to prepare the adefovir dipivoxil product. The invention has simple process, easy operation, safety, environment protection, mild reaction condition and high product purity, uses the most elementary raw materials and common reagents, and can be used for industrialized production. The invention is used for producing the adefovir dipivoxil raw material.
Owner:湖南欧亚药业有限公司
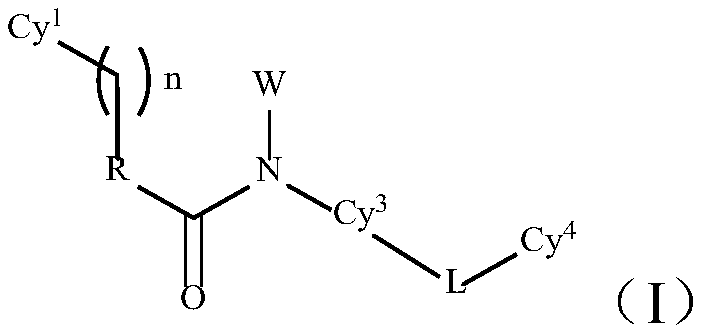
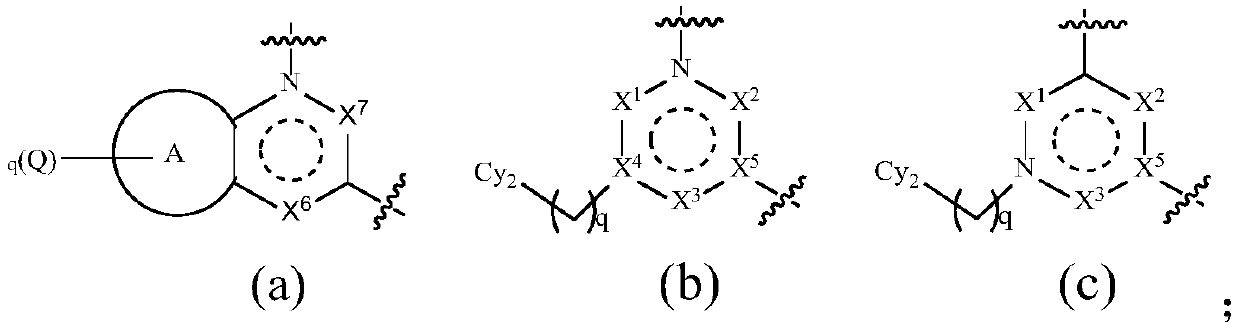
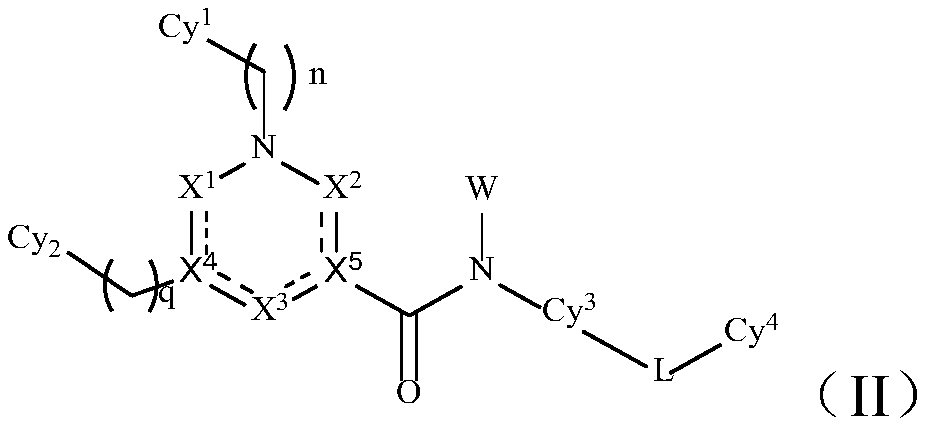
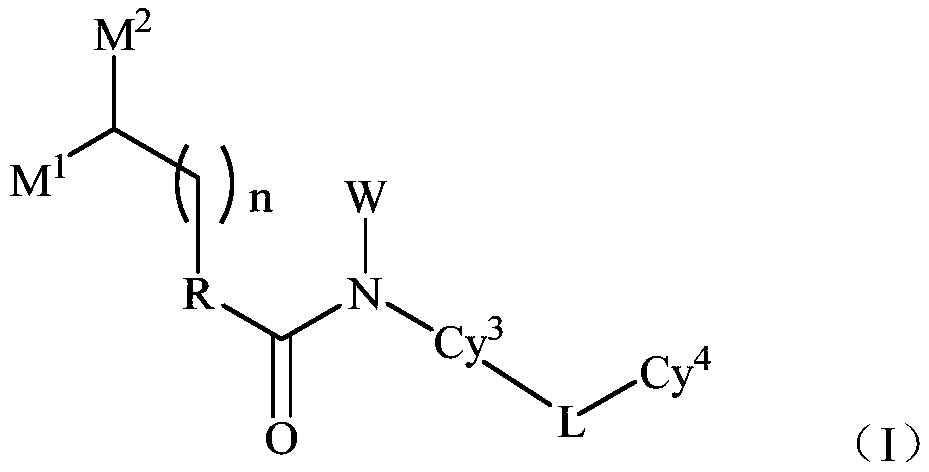
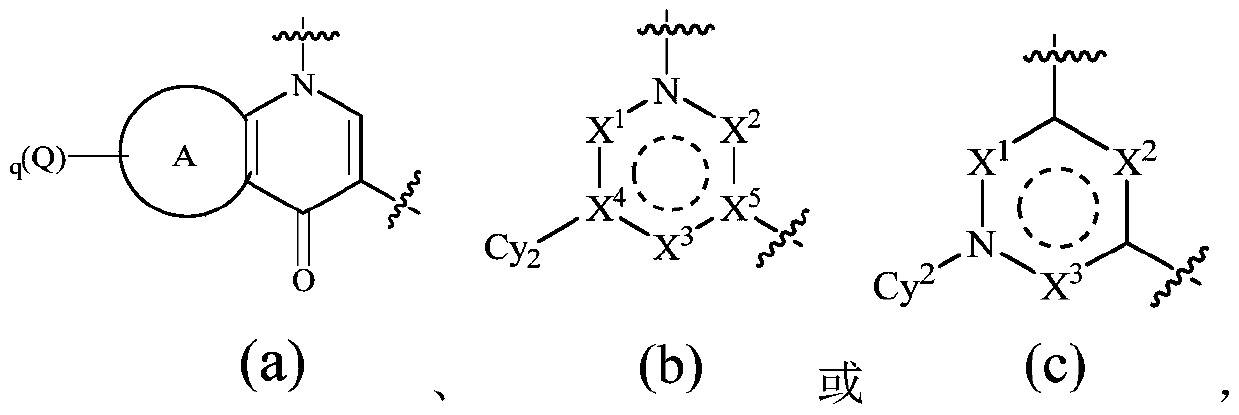
TAM family kinase and/or CSF1R kinase inhibitor and application thereof
ActiveCN110041316AGrowth inhibitionInhibit migrationOrganic active ingredientsSenses disorderAbnormal expressionSrc family kinase
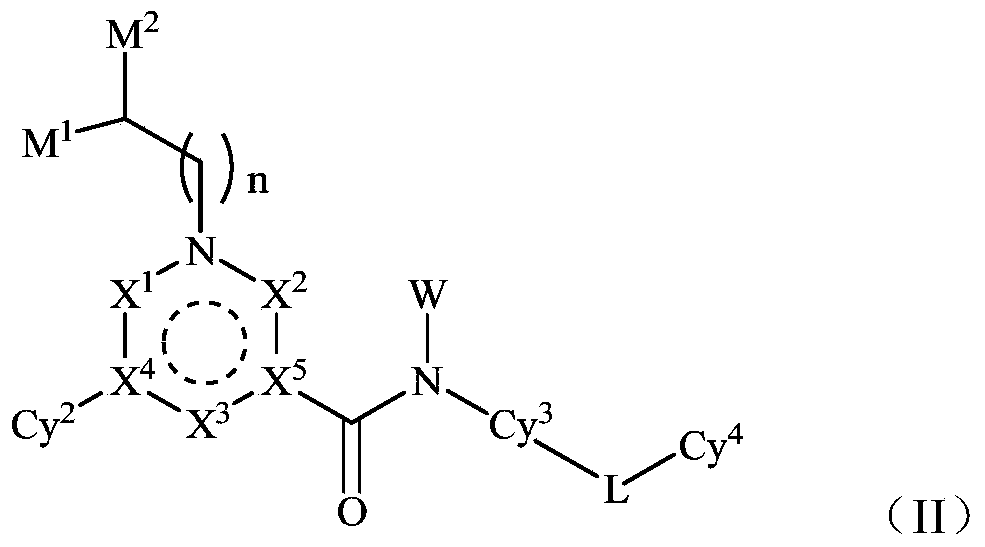
The invention provides a novel inhibitor compound shown in a general formula (I). The compound has good kinase inhibition activity and can be used for preventing and / or treating diseases mediated by abnormal expression of TAM family kinase and / or a ligand thereof. The compound can target CSF1R kinase and can be used for preventing and / or treating diseases mediated by abnormal expression of a TAM family kinase receptor and / or a CSF1R kinase receptor and / or ligands thereof.
Owner:TRANSTHERA SCIENCES (NANJING) INC
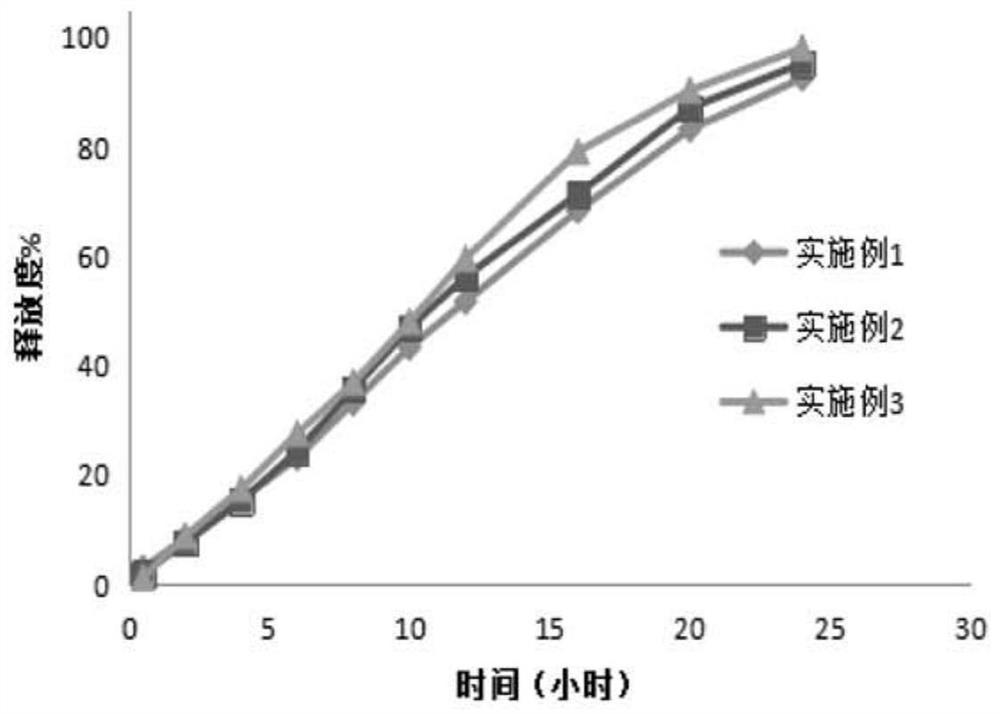
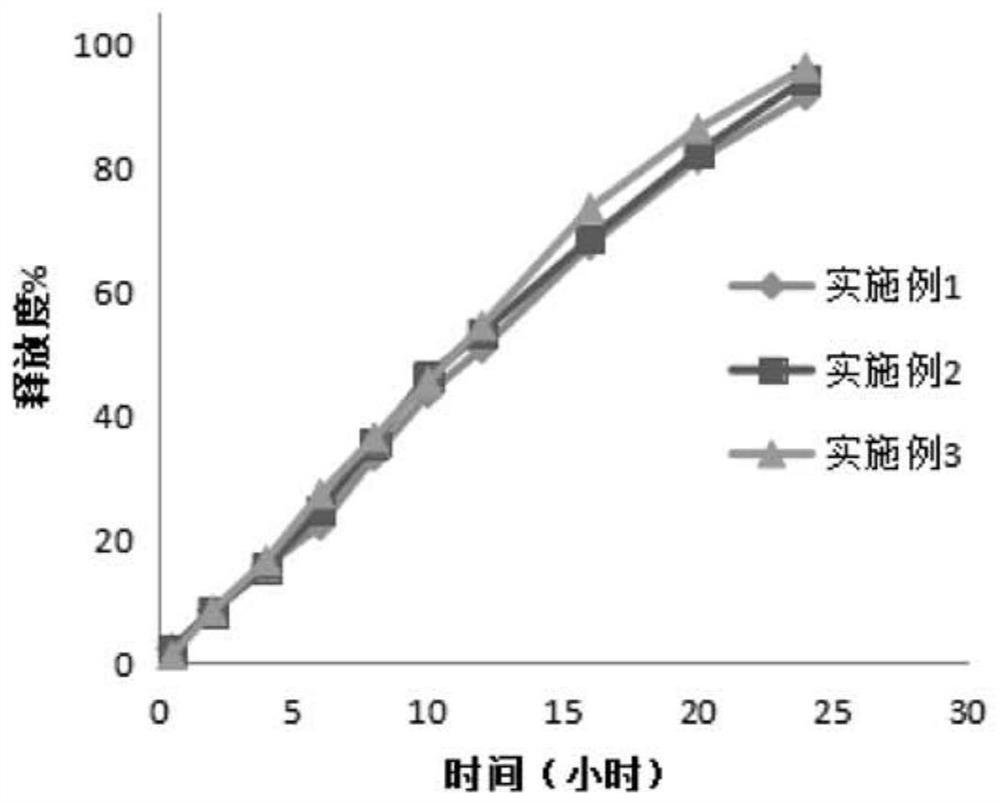
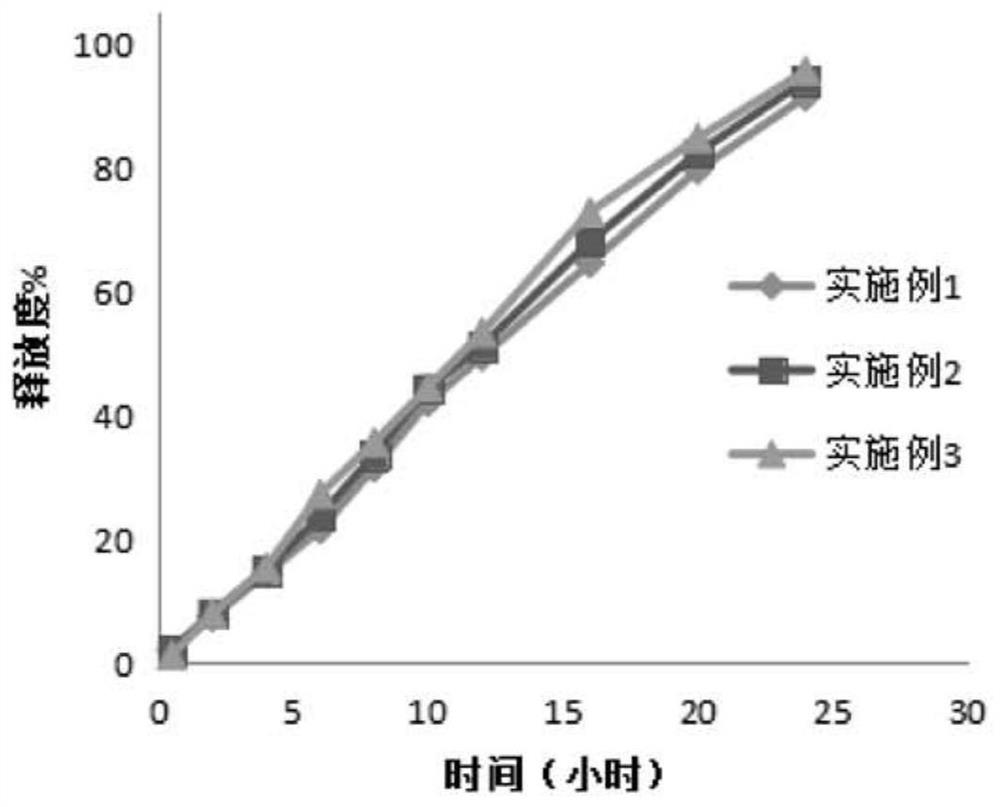
Sustained release tablet containing high water-soluble active ingredients and preparation method thereof
ActiveCN105434386ALow costEasy to buyPill deliveryPharmaceutical non-active ingredientsCarmellose CalciumSustained Release Tablet
The invention belongs to the technical field of pharmaceutical preparations and particularly relates to a sustained release tablet containing high water-soluble active ingredients and a preparation method thereof. The sustained release tablet comprises, by weight percentage, 45%-70% of high water-soluble active ingredients, 0.3%-4% of first sustained-release material, 27%-51% of second sustained-release material, 0-18% of diluent and 0.1%-1.0% of lubricant. The first sustained-release material and the second sustained-release material can be the same or different and are selected from one or more of hydroxypropyl methylcellulose, hydroxy propyl cellulose, sodium carboxymethyl cellulose, carmellose calcium, povidone, carbomer and polyoxyethylene. Compared with the existing sustained release tablet, the sustained release tablet containing the high water-soluble active ingredients is good in compressibility, low in cost and good in quality, and the hardness cannot be reduced after long-term storage.
Owner:华益泰康药业股份有限公司
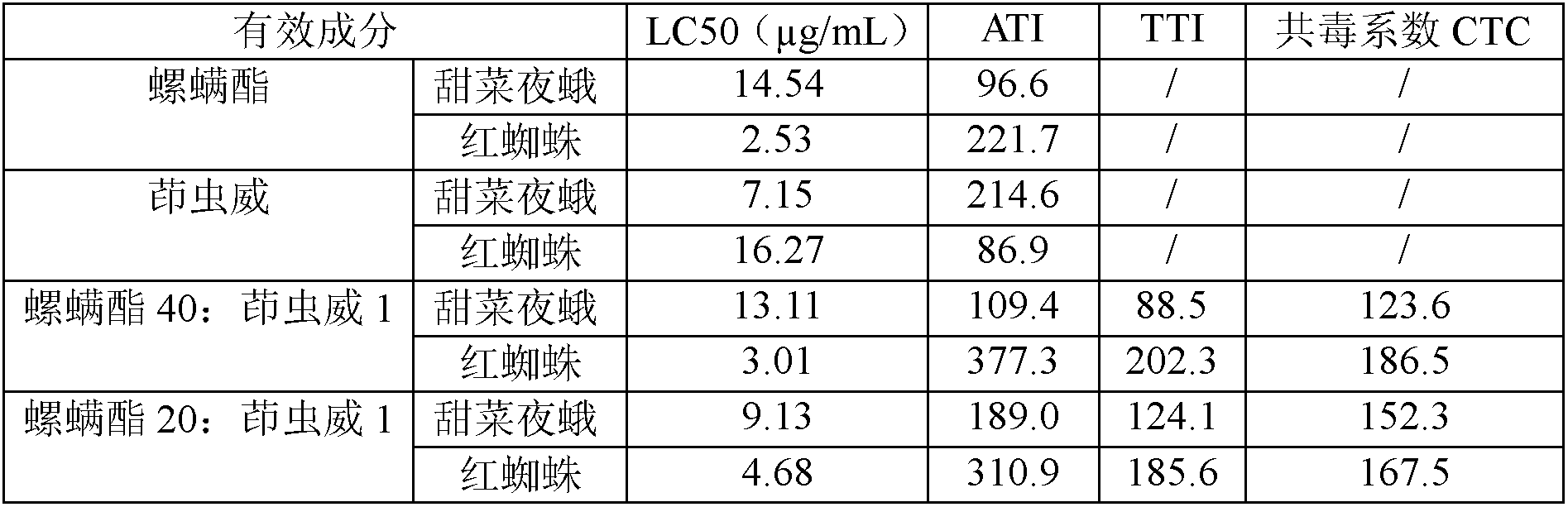
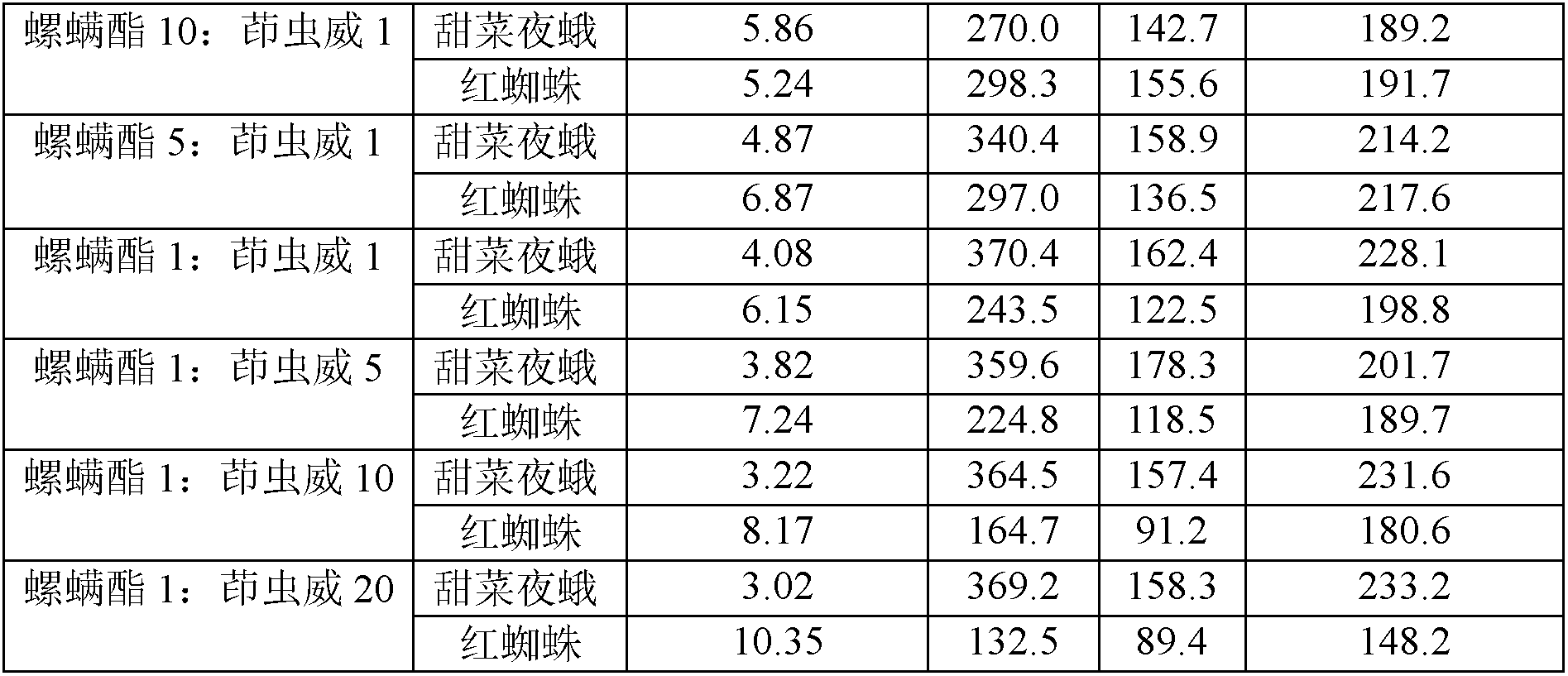
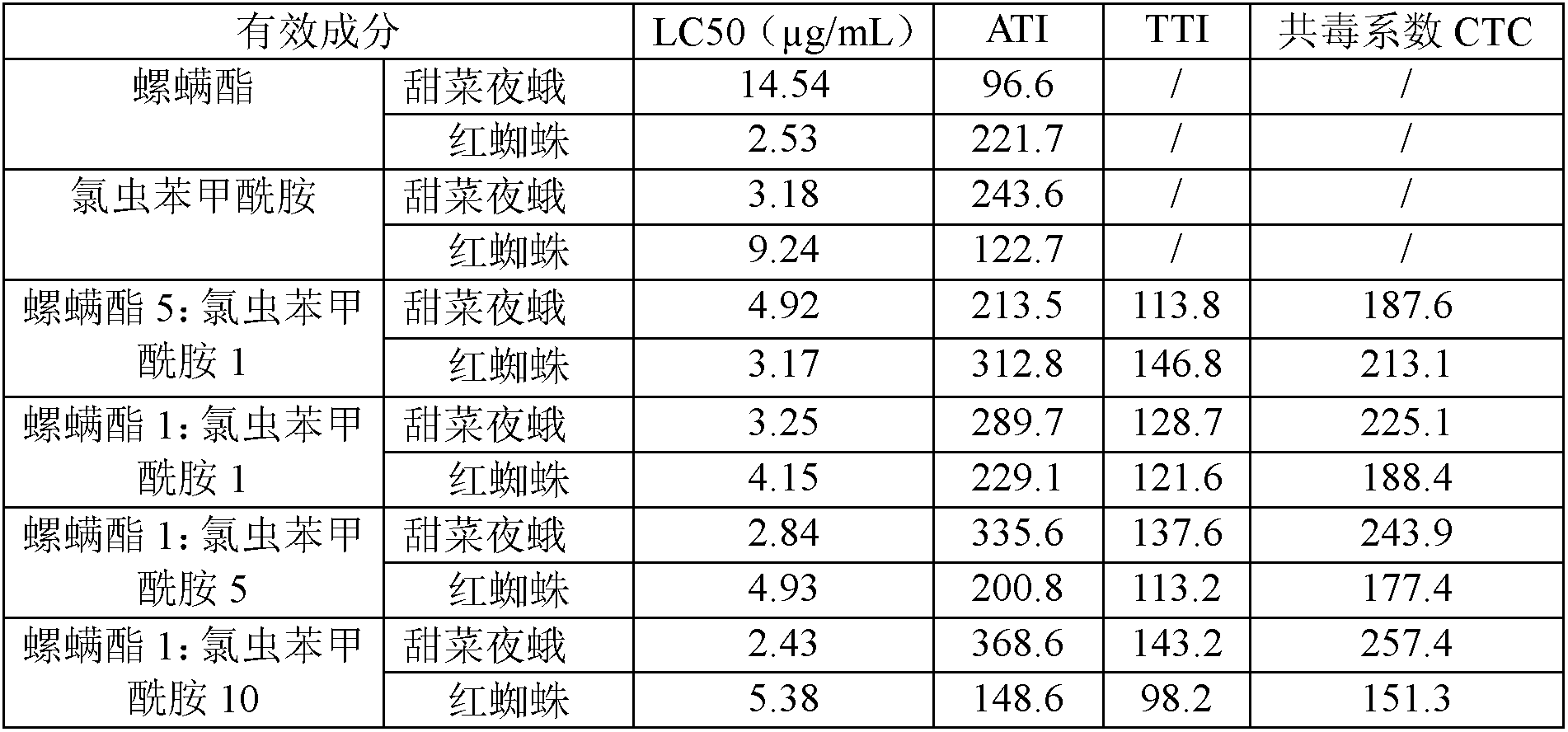
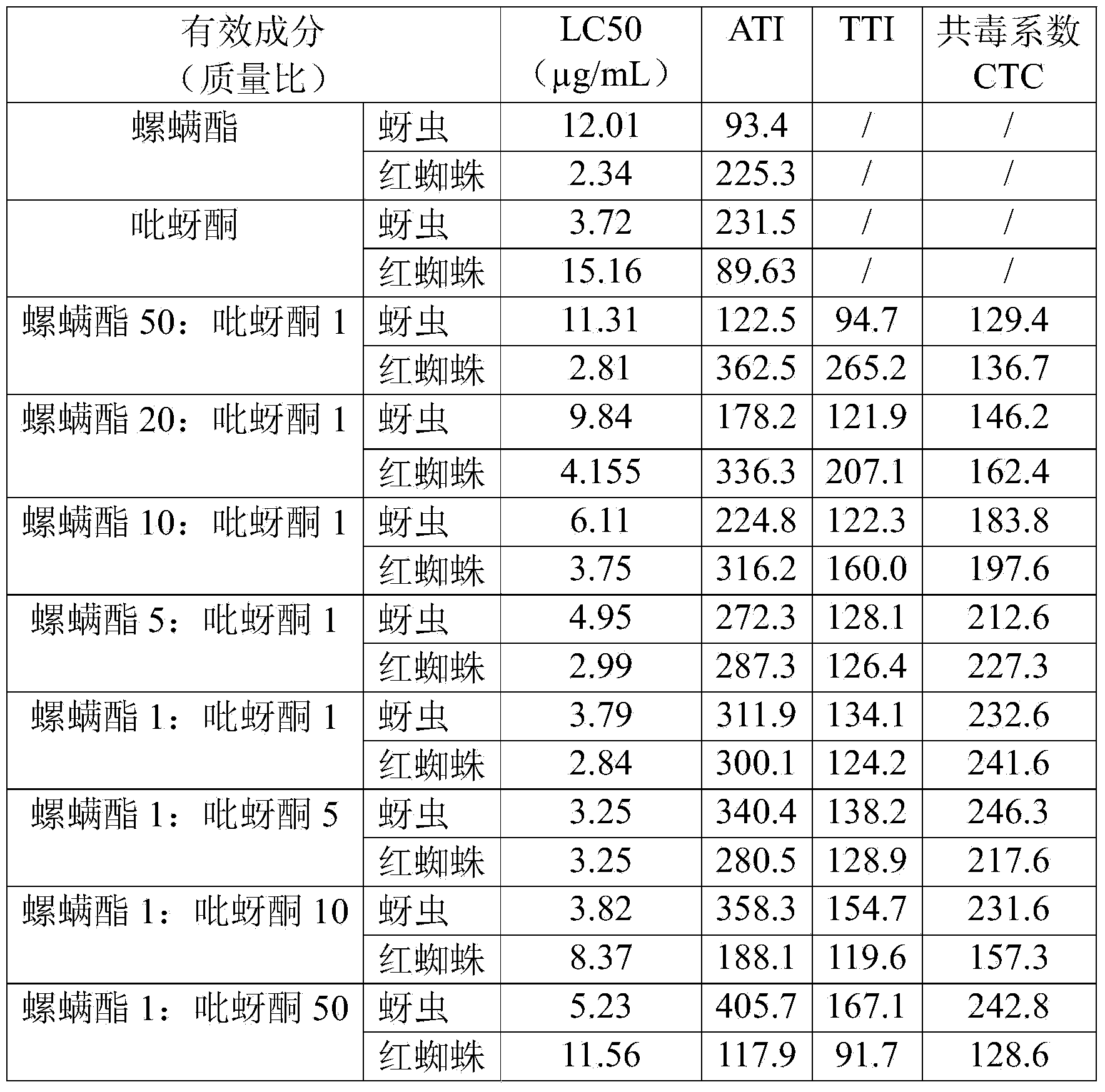
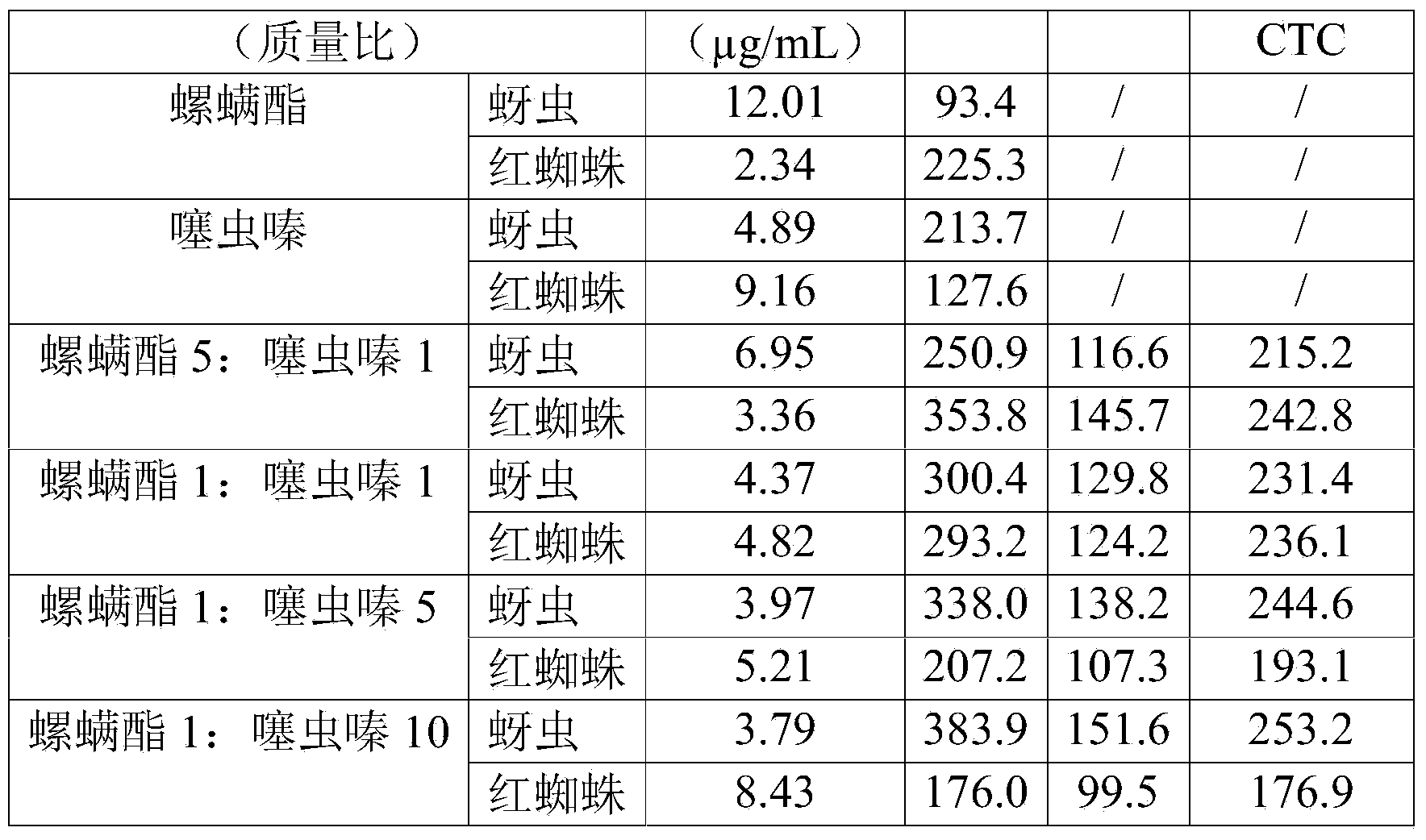
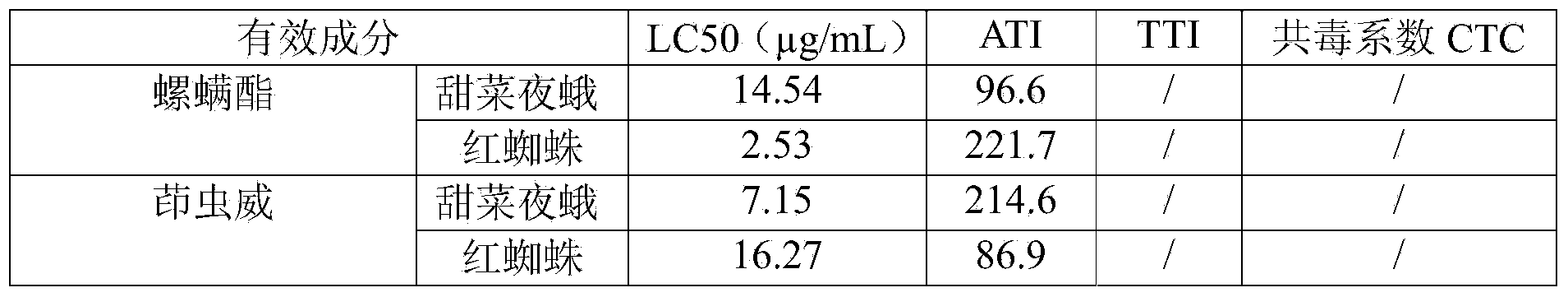
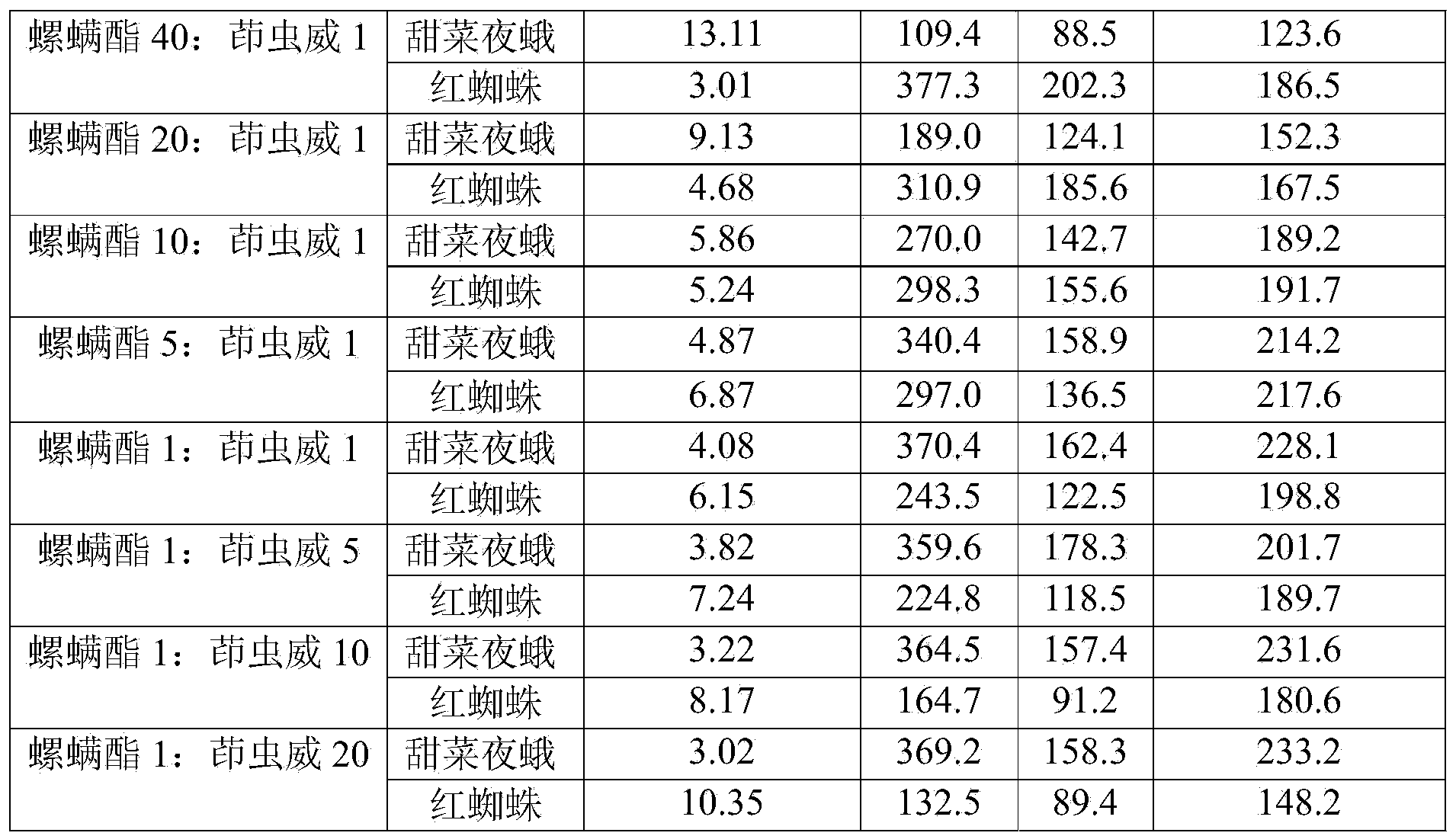
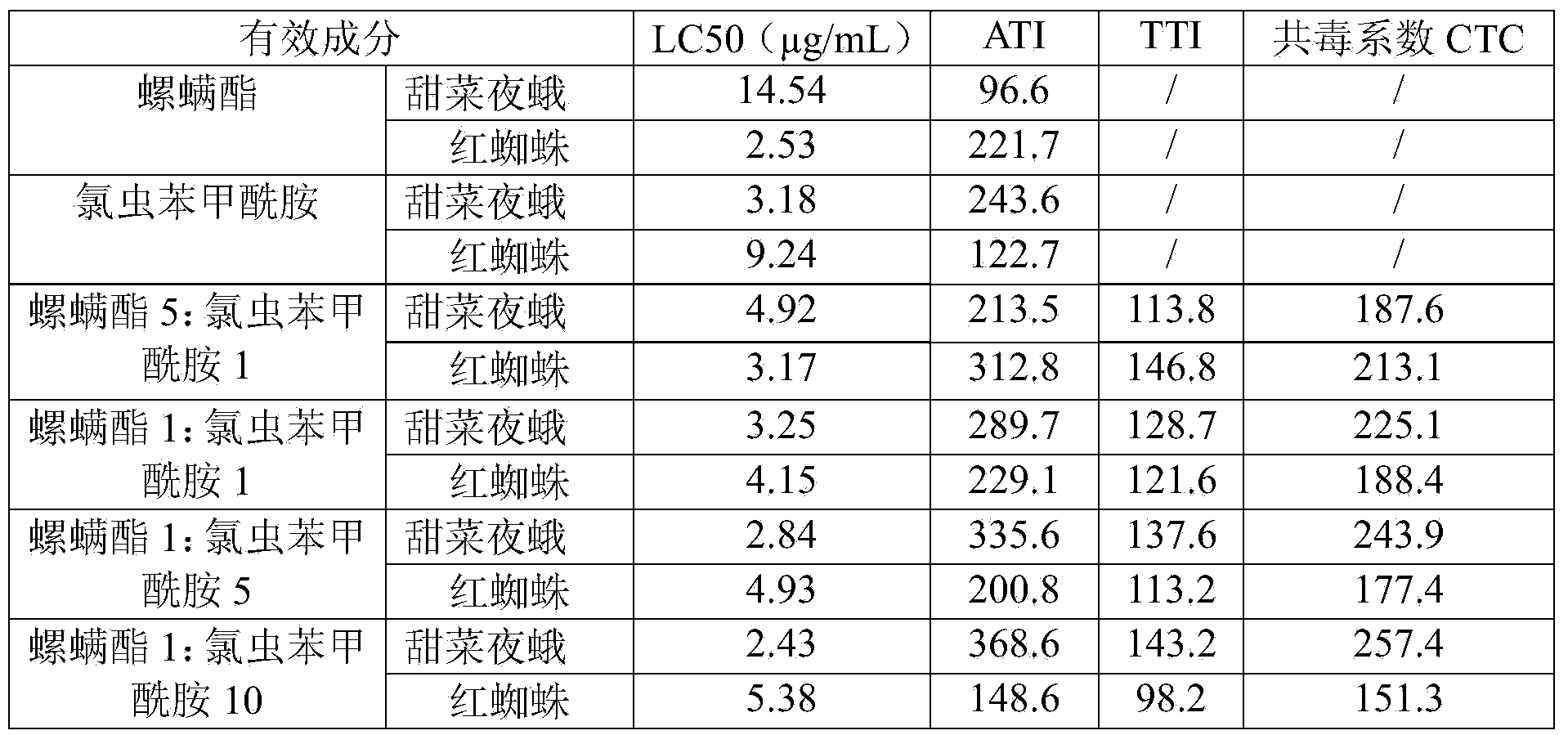
Spirodiclofen-containing compound pesticidal composition and preparation thereof
ActiveCN102696651AExcellent and anti-effectReduce medication burdenBiocideAnimal repellantsFruit treeFlubendiamide
The invention discloses a spirodiclofen-containing compound pesticidal composition. Effective components of the spirodiclofen-containing compound pesticidal composition comprises a component I and a component II, wherein the component I is spirodiclofen; the component II is one of indoxacarb, chlorantraniliprole, tolfenpyrad, chromafenozide, pyridalyl,flubendiamide and chlorfluazuron; and the mass ratio of the component I to the component II is 40:1-1:40. The invention further discloses a preparation which is prepared from the spirodiclofen-containing compound pesticidal composition and is suitable for agricultural use, and application of the spirodiclofen-containing compound pesticidal composition to pest control of crops. The spirodiclofen-containing compound pesticidal composition has a significant synergistic effect and is applied to the pest control of the crops such as fruit trees, cotton, wheat, vegetables, flower, rice and the like, especially with significant effect on control of lepidopteran pests and mite pests.
Owner:YONGNONG BIOSCI
Composite pesticide composition containing spirodiclofen and clothianidin and preparation thereof
ActiveCN103609585AExcellent and anti-effectReduce medication burdenBiocideAnimal repellantsArthropod mouthpartsFruit tree
The invention discloses a composite pesticide composition containing spirodiclofen, the effective components of the composition comprise a component I and a component II, wherein the component I is spirodiclofen, the component II is one component selected from the following components: pymetrozine, thiamethoxam, nitenpyram, clothianidin, dinotefuran, spirotetramat and flonicamid, and the mass ratio of the component I to the component II is 50:1 to 1:50. The invention also discloses a preparation, which is prepared from the composition mentioned above and is suitable for being applied to the agriculture, and an application of the composition mentioned above in the crop pest control. The composite pesticide composition containing spirodiclofen has a prominent synergetic effect, can be used to control pests in fruit trees, cottons, wheat, vegetables, and flowers, and especially has a prominent control effect on pests with piercing-sucking mouthparts and mite pests.
Owner:YONGNONG BIOSCI
Belinostat derivative based on acetic acid, and preparation method and application thereof
ActiveCN107698632AImprove solubilitySolve problems such as side effectsOrganic active ingredientsSugar derivativesAcetic acidSide effect
The invention provides a belinostat derivative based on acetic acid, and a preparation method and application thereof. The belinostat derivative based on acetic acid in the invention uses belinostat with tumor inhibition activity as a mother drug, and a water-soluble substituent is used for improving the dissolvability of belinostat, so the belinostat derivative has good water-solubility, and theproblem of side effects caused by belinostat can be effectively overcome; and the belinostat derivative can be further used for preparation of treatment drugs for tumors. Moreover, the preparation method of the invention has few procedures and simple operation steps and is suitable for large-scale production, etc.
Owner:ZHEJIANG MEDICAL COLLEGE
Sustained-release tablet containing dual-layer tablet core
InactiveCN105832689ALow costEasy to buyOrganic active ingredientsNervous disorderSustained Release TabletPlasticizer
The invention belongs to the technical field of medicine preparations, and particularly relates to a sustained-release tablet containing a dual-layer tablet core. The sustained-release tablet comprises the dual-layer tablet core and an enteric layer, wherein the dual-layer table core comprises a medicated layer and a blank layer; the enteric layer coats the dual-layer tablet core; and a plasticizer in the blank layer is one or more of hexadecanol or polyethylene glycol. A specific plasticizer is added to the blank layer, and the blank layer is not separated from the medicated layer within a certain period of time, so that stable medicine release is achieved, and burst release is avoided. Compared with an existing sustained-release tablet, the sustained-release tablet is relatively low in cost and invariable in quality after being stored for a long period of time.
Owner:华益泰康药业股份有限公司
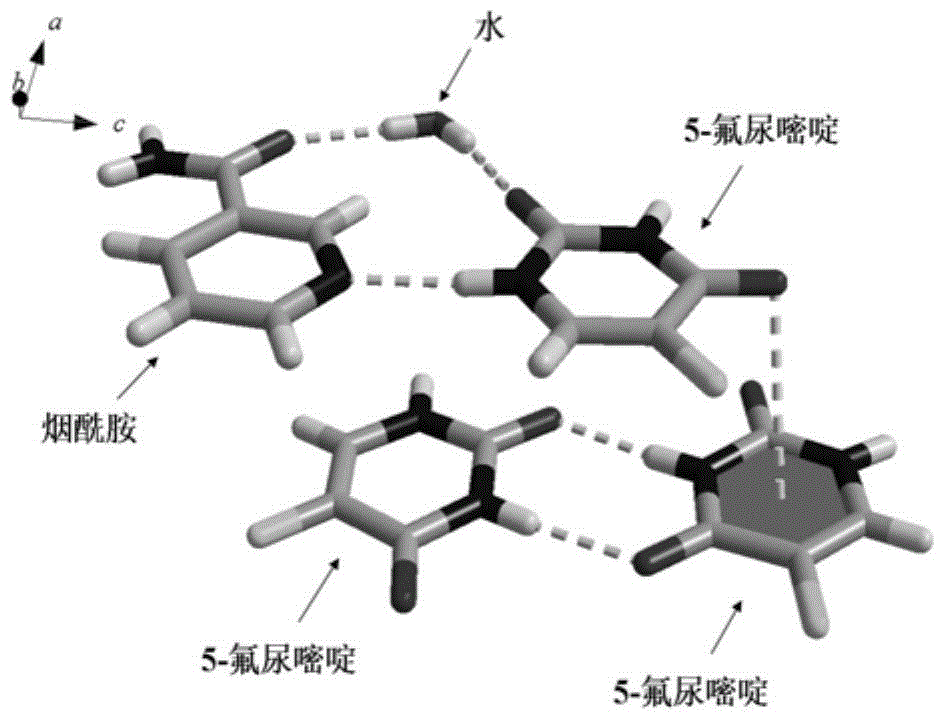
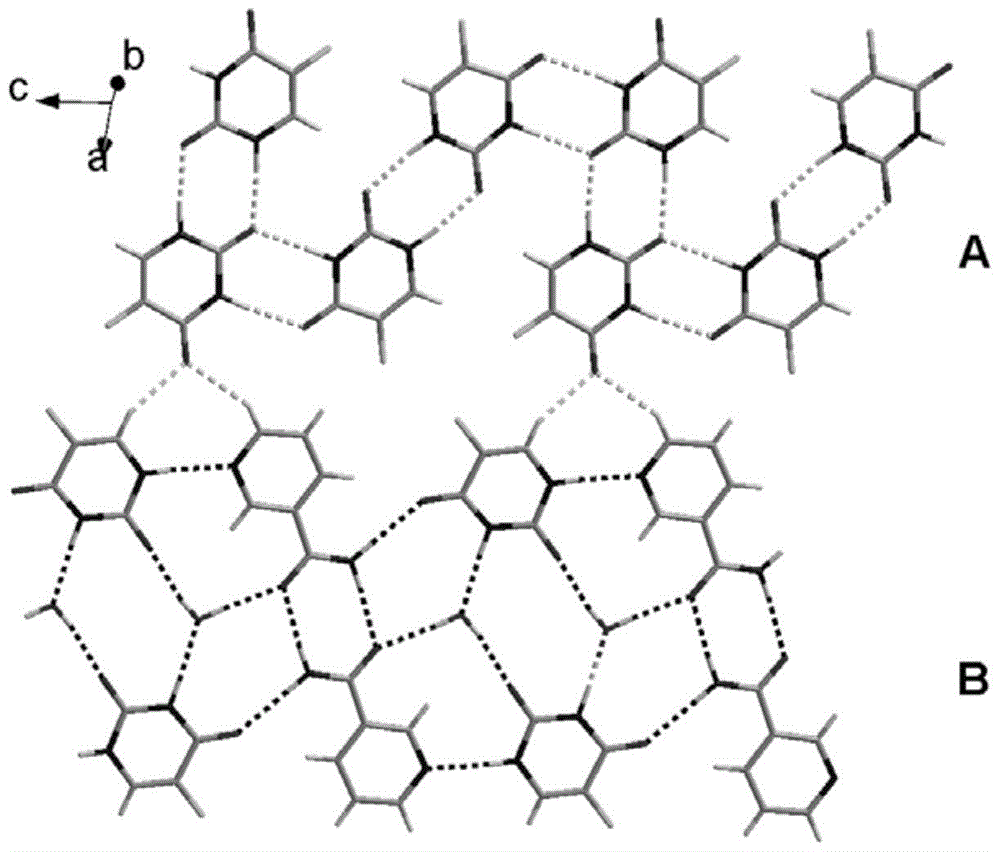
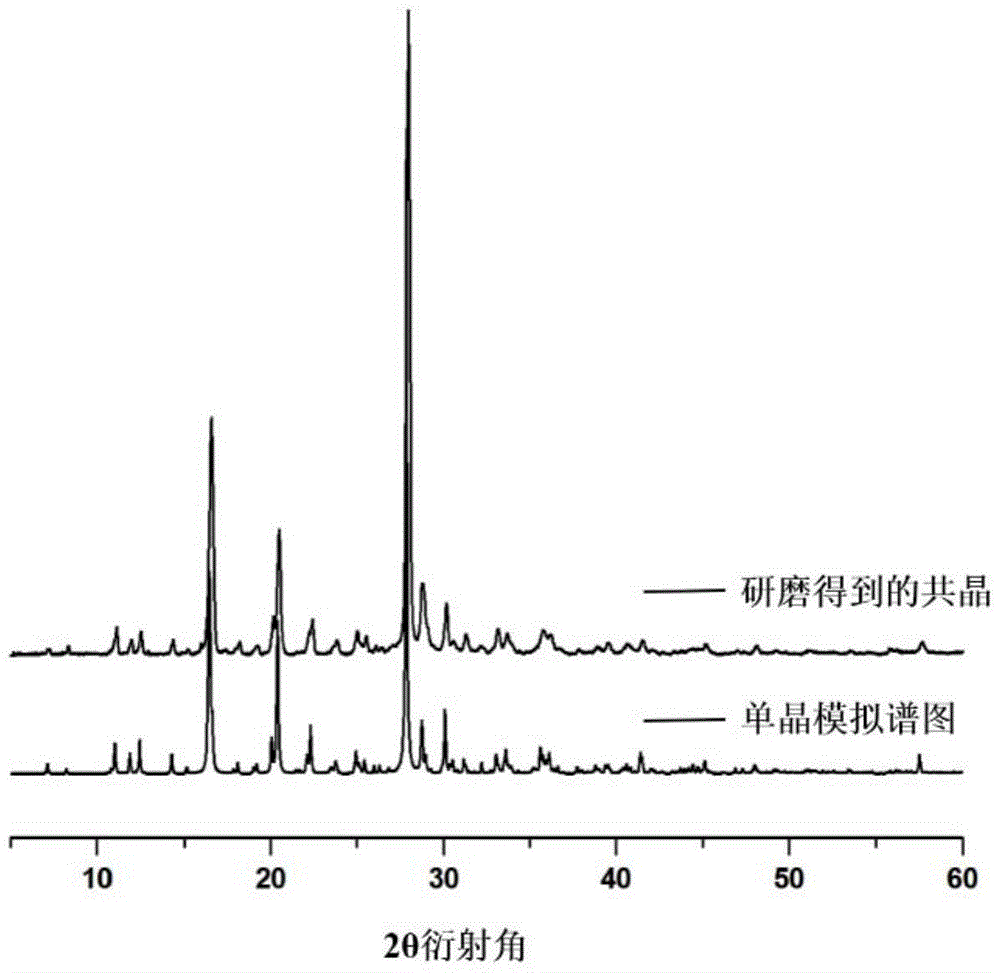
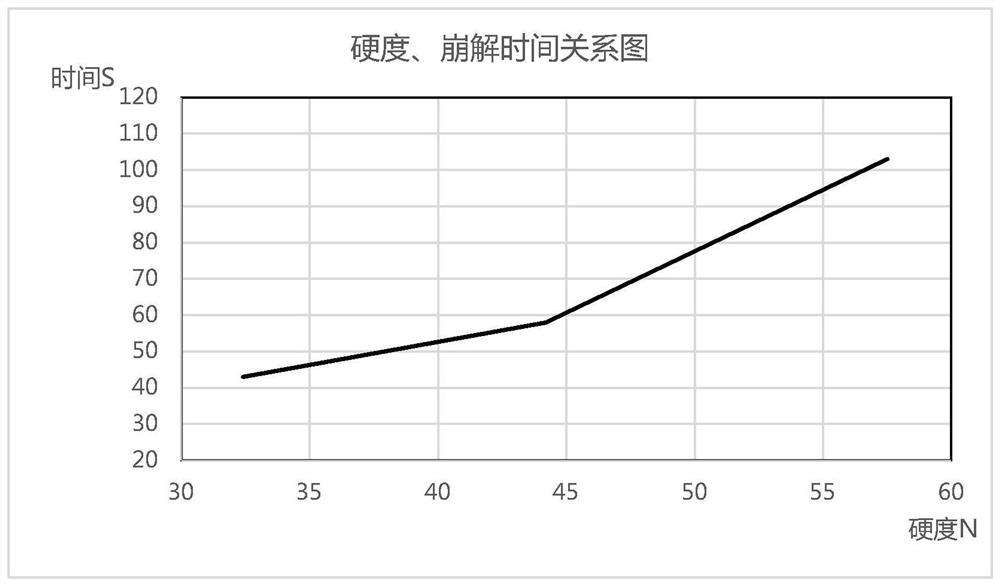
Medicine preparation containing 5-fluorouracil drug eutectic with nicotinamide as precursor and preparation method of medicine preparation
ActiveCN105732517ASignificant effectInhibit synthesisOrganic active ingredientsOrganic chemistryUse medicationExtended release tablets
The invention discloses a medicine preparation containing 5-fluorouracil drug eutectic with nicotinamide as a precursor and a preparation method of the medicine preparation.5-fluorouracil raw material medicine is selected as medicine API, nicotinamide serves as the medicine precursor, a liquid-phase assisted grinding method is adopted, the high-purity 5-fluorouracil drug eutectic is obtained, and the effect that the medicine can be more stable can be achieved; in addition, the invention further provides the medicine preparation containing the 5-fluorouracil drug eutectic, the medicine preparation comprises tablets, slow-release tablets, microspheres, micro-capsules, suppository, emulsion, a film agent and an injection agent so that stability and the curative effect of the 5-fluorouracil drug eutectic with the nicotinamide as the precursor can be better improved, different medicine application modes are utilized, and possibility of fully playing the treatment function of medicine and lowering or avoiding adverse reactions is provided.Auxiliaries applied to the preparation method of the preparation are easy to buy in the market and low in price, therefore cost is quite low, and the medicine application burden of patients can be remarkably lowered.
Owner:HARBIN MEDICAL UNIVERSITY
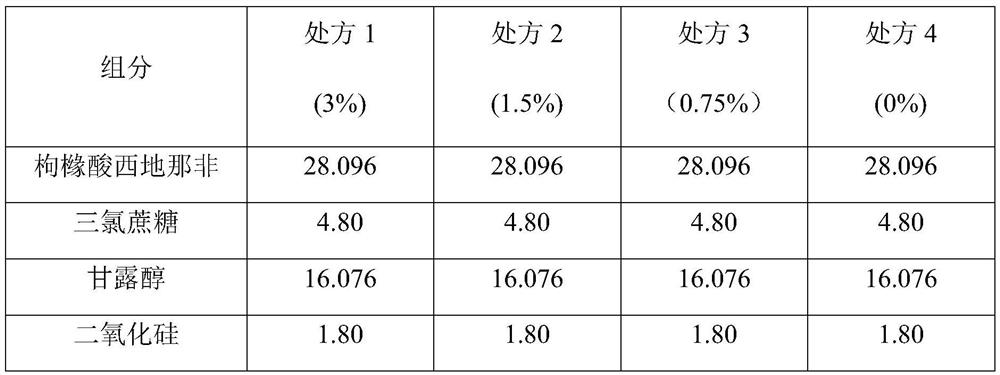
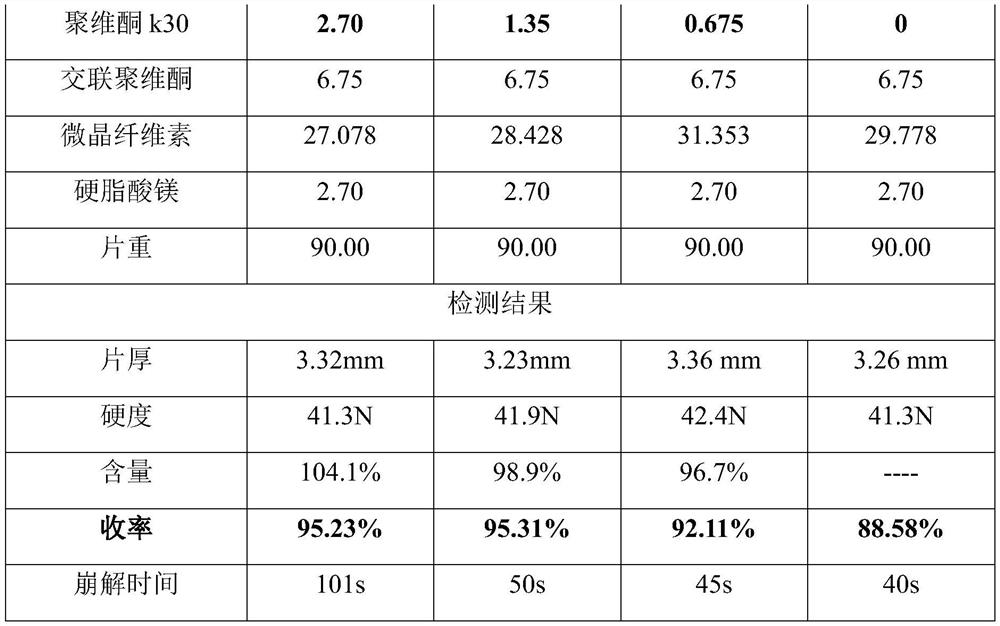
Pharmaceutical composition containing sildenafil citrate, and preparation method and application thereof
ActiveCN113413388ALow costReduce medication burdenOrganic active ingredientsInorganic non-active ingredientsMannitolCroscarmellose sodium
The invention provides a pharmaceutical composition containing sildenafil citrate, which adopts a wet granulation process and comprises the following components in percentage by weight: 3 to 45 percent of sildenafil citrate, 0.5 to 5 percent of adhesive, 2 to 20 percent of sweetening agent, 3 to 20 percent of disintegrating agent, 0.5 to 3 percent of flow aid, 0.5 to 5 percent of lubricating agent, and the balance of filler. The filler is selected from a mixture of microcrystalline cellulose and mannitol; the adhesive is selected from one or a mixture of more than one of povidone, hydroxypropyl methylcellulose and hydroxy propyl cellulose; the disintegrating agent is selected from one or a mixture of more than one of polyvinylpolypyrrolidone, croscarmellose sodium and low-substituted hydroxy propyl cellulose, and the pharmaceutical composition is a low-dose dispersible tablet of sildenafil citrate and has the advantages of low cost, easiness in preparation, high dissolution speed and short disintegration time.
Owner:SHANGHAI AUSON PHARM CO LTD
Novel quinoline derivative inhibitor
ActiveCN110857293AGrowth inhibitionInhibit migrationOrganic active ingredientsSenses disorderReceptorTyrosine
Owner:TRANSTHERA SCIENCES (NANJING) INC
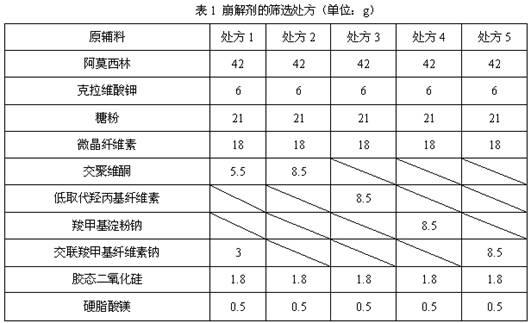
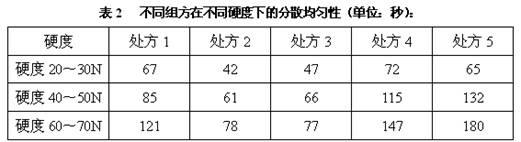
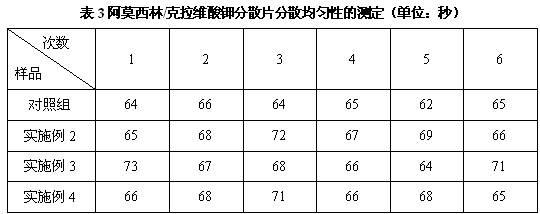
Dispersible tablet containing amoxicillin and potassium clavulanate
ActiveCN102600141AImprove dispersion uniformityHigh dissolution rateAntibacterial agentsPill deliveryWestern medicineLow-substituted hydroxypropylcellulose
The invention belongs to the technical field of western medicine preparations, and particularly relates to a dispersible tablet containing amoxicillin and potassium clavulanate. The dispersible tablet comprises the following components in parts by weight: 420 parts of amoxicillin, 60 parts of potassium clavulanate, 350-400 parts of filler, 80-100 parts of low-substituted hydroxypropyl cellulose, 1-20 parts of colloidal silicon dioxide and 5-8 parts of magnesium stearate. For the dispersible tablet, both the dispersing uniformity and dissolution achieve the technological effects of the existing product, simultaneously, and the hardness of the pressed tablet is within 50-70N, so that the problems of insufficient hardness and difficulty in common packaging existing in products on the market are solved.
Owner:NANJING CHENGONG PHARM CO LTD
External traditional Chinese medicine ointment for treating scapulohumeral periarthritis
InactiveCN104758845ASignificant effectReduce medication burdenAntipyreticTetracycline active ingredientsDandelionCarthamus
The invention discloses an external traditional Chinese medicine ointment for treating scapulohumeral periarthritis. The external traditional Chinese medicine ointment is prepared from the following traditional Chinese medicine raw materials in parts by weight: 20-30 parts of spreading hedyotis herb, 8-12 parts of cassia twig, 12-15 parts of incised notopterygium, 15-20 parts of white peony root, 18-25 parts of clematis root, 8-12 parts of zedoary rhizome, 8-12 parts of cape jasmine fruit, 3-5 parts of borax, 5-6 parts of rhizoma corydalis, 8-12 parts of weeping forsythia capsule, 10-12 parts of root of lobed kudzuvine, 8-12 parts of mongolian dandelion herb, 8-12 parts of safflower, 10-15 parts of schizonepeta spike, 2-5 parts of oxytetracycline powder, 8-12 parts of fruit-spike of common selfheal, 8-12 parts of barbed skullcap herb, 8-12 parts of Chinese thorowax root, 8-12 parts of milkvetch root, 8-10 parts of dried tangerine peel, 10-12 parts of flower of Japanese pagodatree, 12-15 parts of tree peony bark and 4-6 parts of talc powder. The external traditional Chinese medicine ointment disclosed by the invention is dialectical in compatibility of all raw medicinal materials to achieve the effects of clearing away heat and toxic materials, promoting blood circulation to dispel blood stasis, invigorating spleen and kidney, regulating qi to relieve epigastric distention, diminishing inflammation and relieving pains and the like, has a significant curative effect on scapulohumeral periarthritis patients, is high in cure rate and quick in response, and does not have toxic or side effects.
Owner:张先雷
Compound pesticide composition containing clodinafop-propargyl and picloram or salts thereof, preparation thereof and applications of the preparation
InactiveCN104115834AReduce the dosageReduce medication burdenBiocideAnimal repellantsCOMPONENT IIFlucarbazone
A compound pesticide composition containing picloram or salts thereof is disclosed. Effective components of the composition are a component I and a component II. The component I is the picloram or the salts thereof. The component II is one of flumetsulam, pyribenzoxim, carfentrazone-ethyl, topramezone, florasulam, flucarbazone, clodinafop-propargyl or metamifop. The weight ratio of the component I to the component II is 60:1-1:60. A preparation prepared from the composition and suitable for use in agriculture, and applications of the preparation in weed control are disclosed. The composition has obvious synergistic effects, achieves control of broadleaved weeds and annual grassy weeds, reduces the pesticide using amount, relieves pesticide burden of farmers, reduces pollution to the environment, and has a wide development and popularization prospect.
Owner:YONGNONG BIOSCI
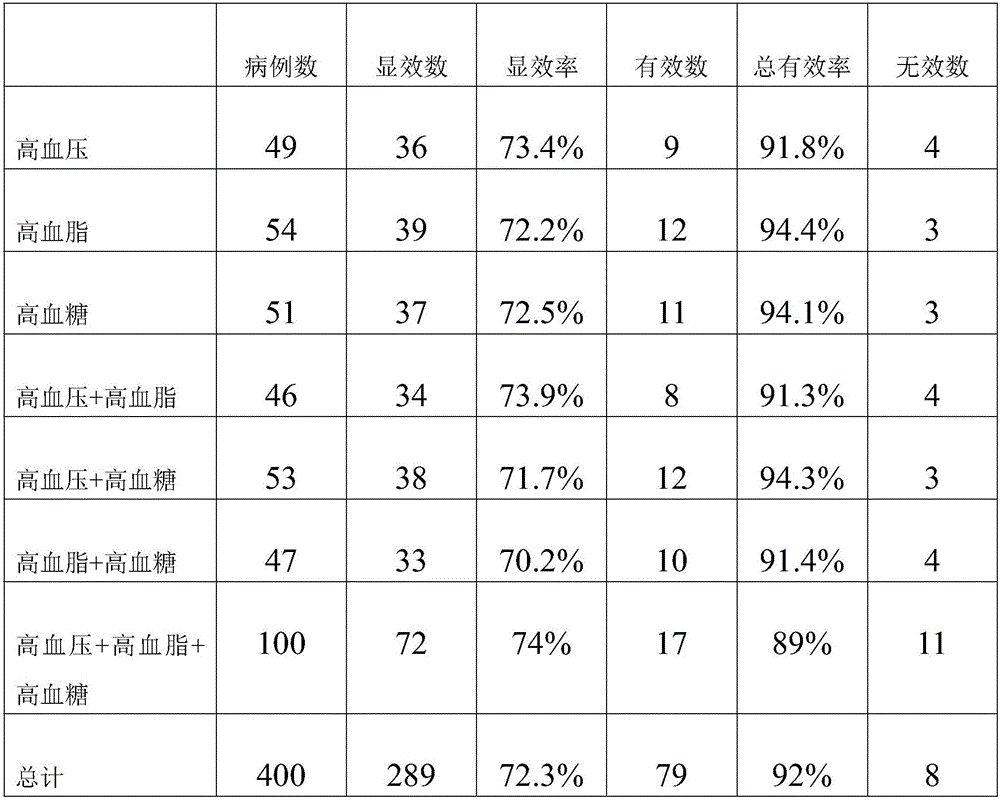
Traditional Chinese medicine composition for treating hypertension, hyperlipidemia and hyperglycemia and preparing method and application thereof
InactiveCN105833055AQuick cureEasy to carryMetabolism disorderGinkgophyta medical ingredientsAcute hyperglycaemiaCure rate
The invention relates to a traditional Chinese medicine composition for treating hypertension, hyperlipidemia and hyperglycemia and a preparing method and application thereof. The traditional Chinese medicine composition is prepared from the roots of red-rooted salvia, radix notoginseng, ginseng, flos rosae rugosae, rhizoma alismatis, ginkgo leaves, hawthorn, rhizoma chuanxiong, lotus leaves, safflowers, gastrodia elata and rhodiola rosea. The traditional Chinese medicine composition has the advantages of being lasting in effect and high in cure rate; the effective-ingredient extraction ratio of the preparing method is high, and capsules prepared with the method are convenient to take.
Owner:贾桥
Alpha-glucosidase inhibitor and application thereof in hypoglycemic drugs or functional foods
ActiveCN111195247AGood biocompatibilityImprove targetingOrganic active ingredientsMetabolism disorderAdjuvantPharmaceutical drug
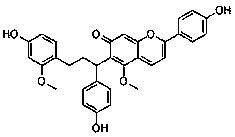
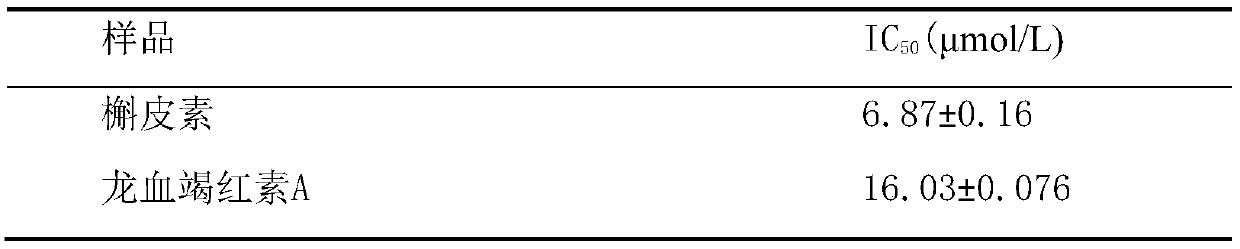
The invention provides a new application of dracorhodin A, including an application of dracorhodin A in preparation of hypoglycemic drugs, health-care products or functional foods, and further including an application of dracorhodin A in preparation of an alpha-glucosidase activity inhibitor. The application creatively finds that dracorhodin A can inhibit the activity of alpha-glucosidase, and theinhibitory activity of dracorhodin A is far higher than that of acarbose serving as a clinical drug. The invention also provides a hypoglycemic composition, which comprises dracorhodin A and pharmaceutic adjuvants or health-care product adjuvants, and is used as a pharmaceutical preparation or a health-care product preparation.
Owner:XISHUANGBANNA TROPICAL BOTANICAL GARDEN CHINESE ACAD OF SCI
Dental implant with osteogenesis-anti-inflammation-blood glucose three-dimensional response structure and preparation method thereof
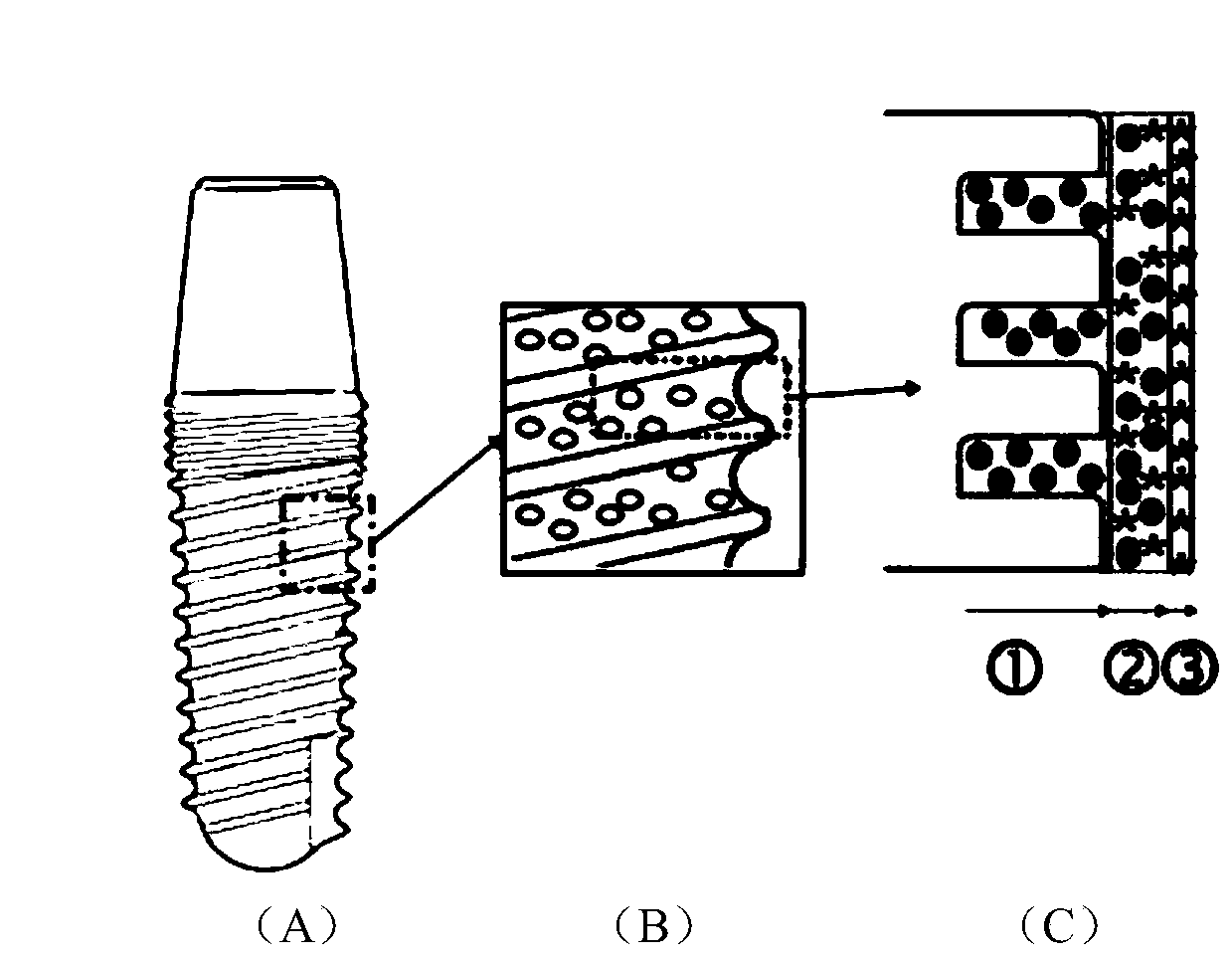
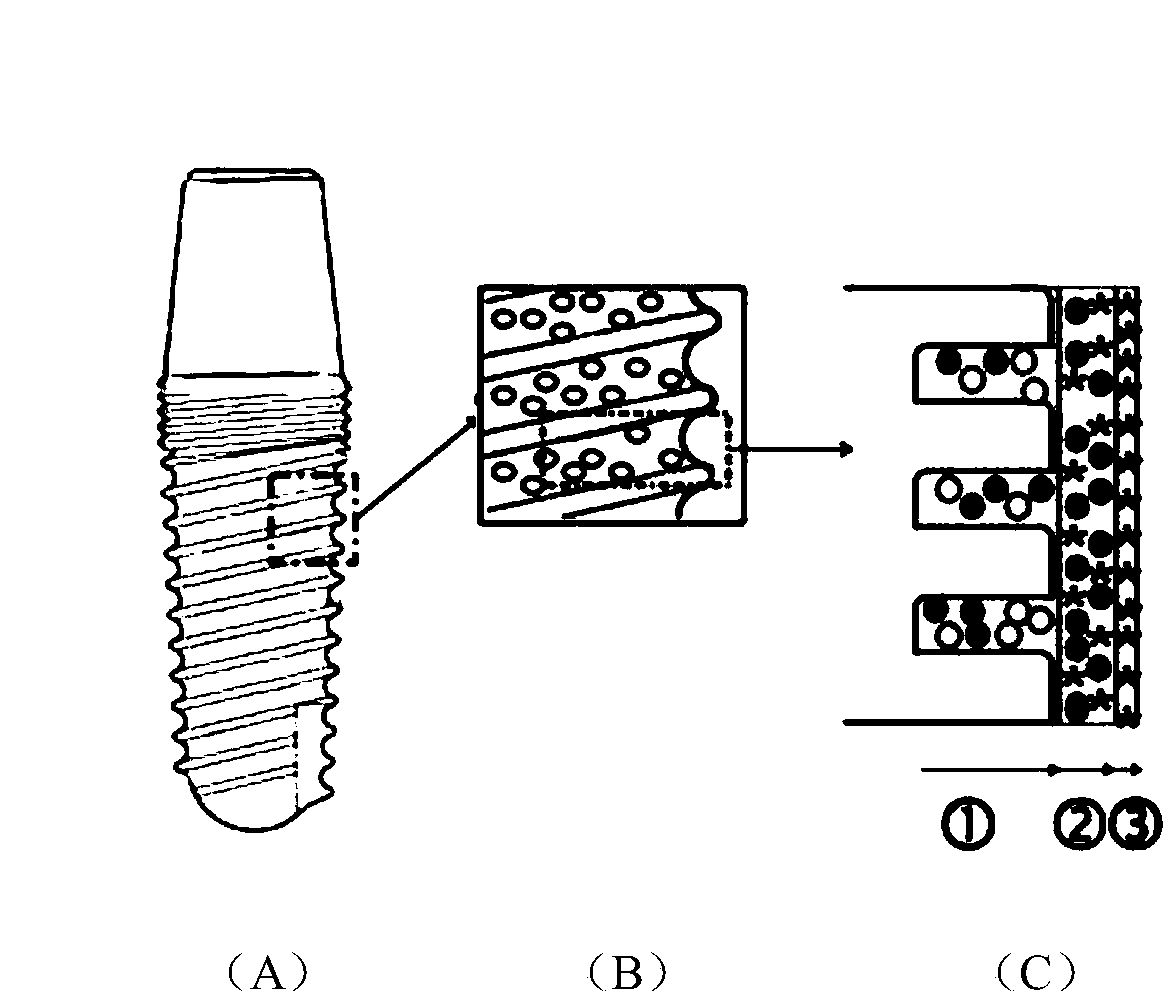
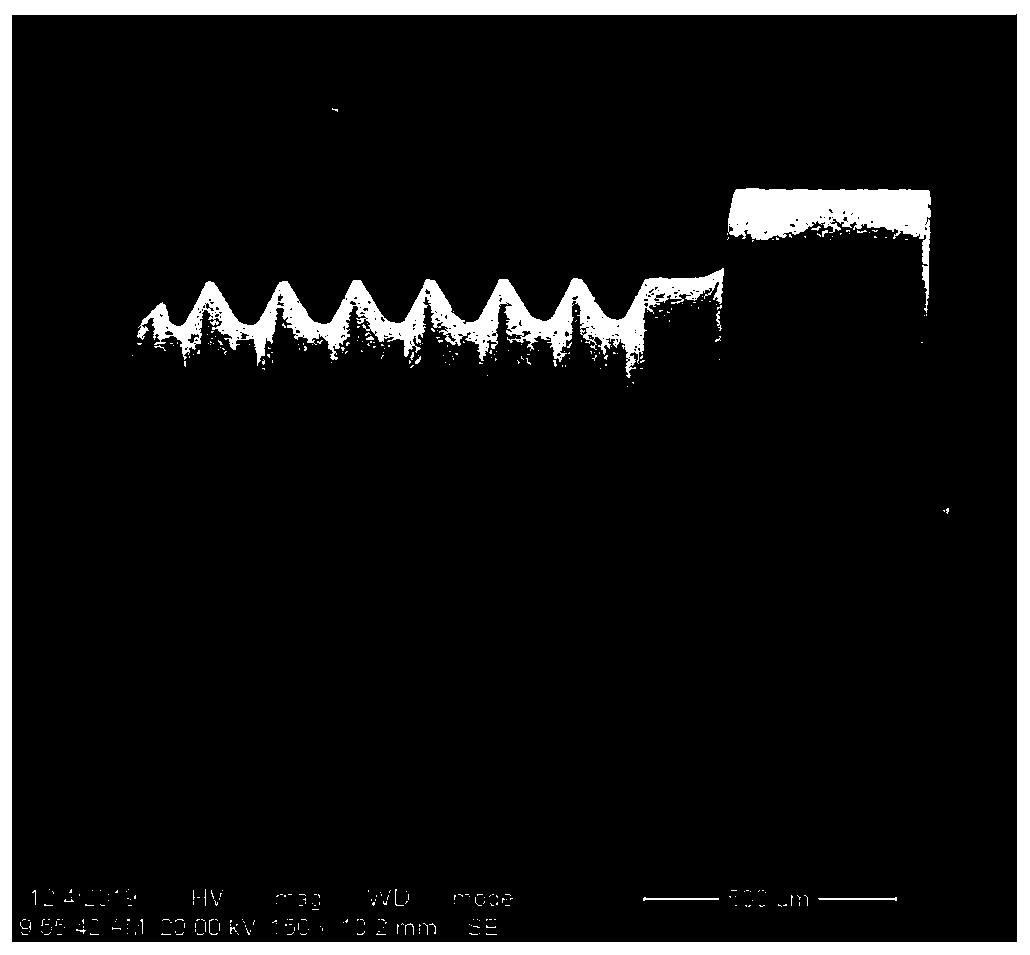
ActiveCN110859997AFacilitated releaseEasy to integrateTissue regenerationCoatingsOsseointegrationQuaternized chitosan
The invention provides a dental implant with an osteogenesis-anti-inflammation-blood glucose three-dimensional response structure. The dental implant consists of a dental implant and a drug controlled-release system with an osteogenesis-anti-inflammation-blood glucose three-dimensional response structure, and the drug controlled-release system is composed of an osteogenesis layer, an anti-inflammation layer and a blood glucose sensing layer. The osteogenesis layer is composed of chitosan hydrogel and nano-hydroxyapatite dispersed in the chitosan hydrogel, the anti-inflammation layer is composed of cross-linked quaternized chitosan hydrogel and anti-inflammation substances and glucose oxidase which are dispersed in the cross-linked quaternized chitosan hydrogel, and the blood glucose sensing layer is a coating composed of glucose oxidase. The surface of the dental implant is provided with a nanopore structure, the nanopore structure of the dental implant is filled with the osteogenesislayer of the drug controlled-release system, the osteogenesis layer coats the dental implant, the anti-inflammation layer coats the osteogenesis layer, and the blood glucose sensing layer coats the anti-inflammation layer. The dental implant can promote osseointegration in a hyperglycemia state, provides a new anti-inflammation treatment technology for patients with diabetes mellitus, and can meetthe clinical requirements of dental implant repair of the patients with diabetes mellitus.
Owner:SICHUAN UNIV
Traditional Chinese medicine mixture for relieving stomach diseases and preparation method thereof
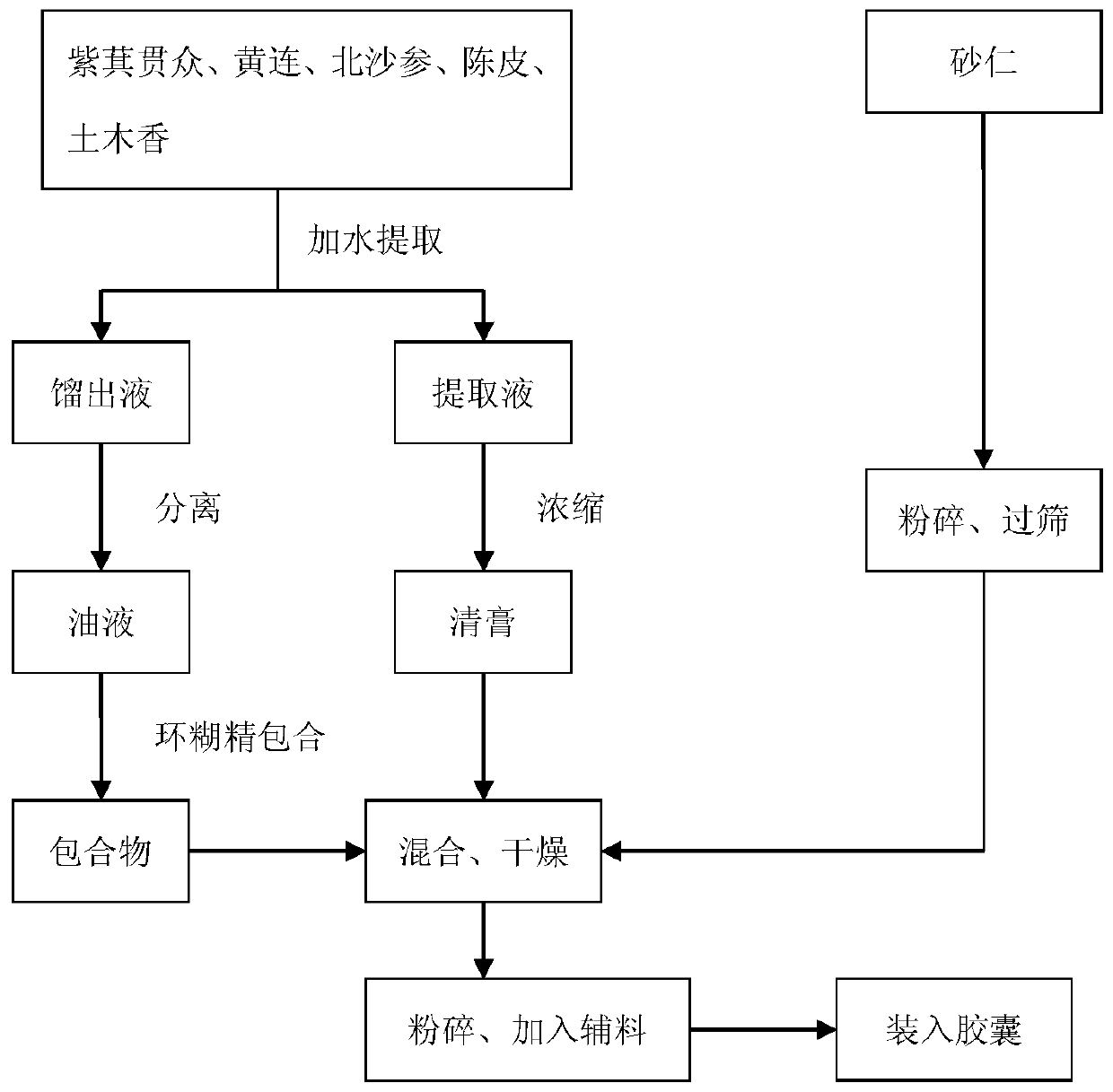
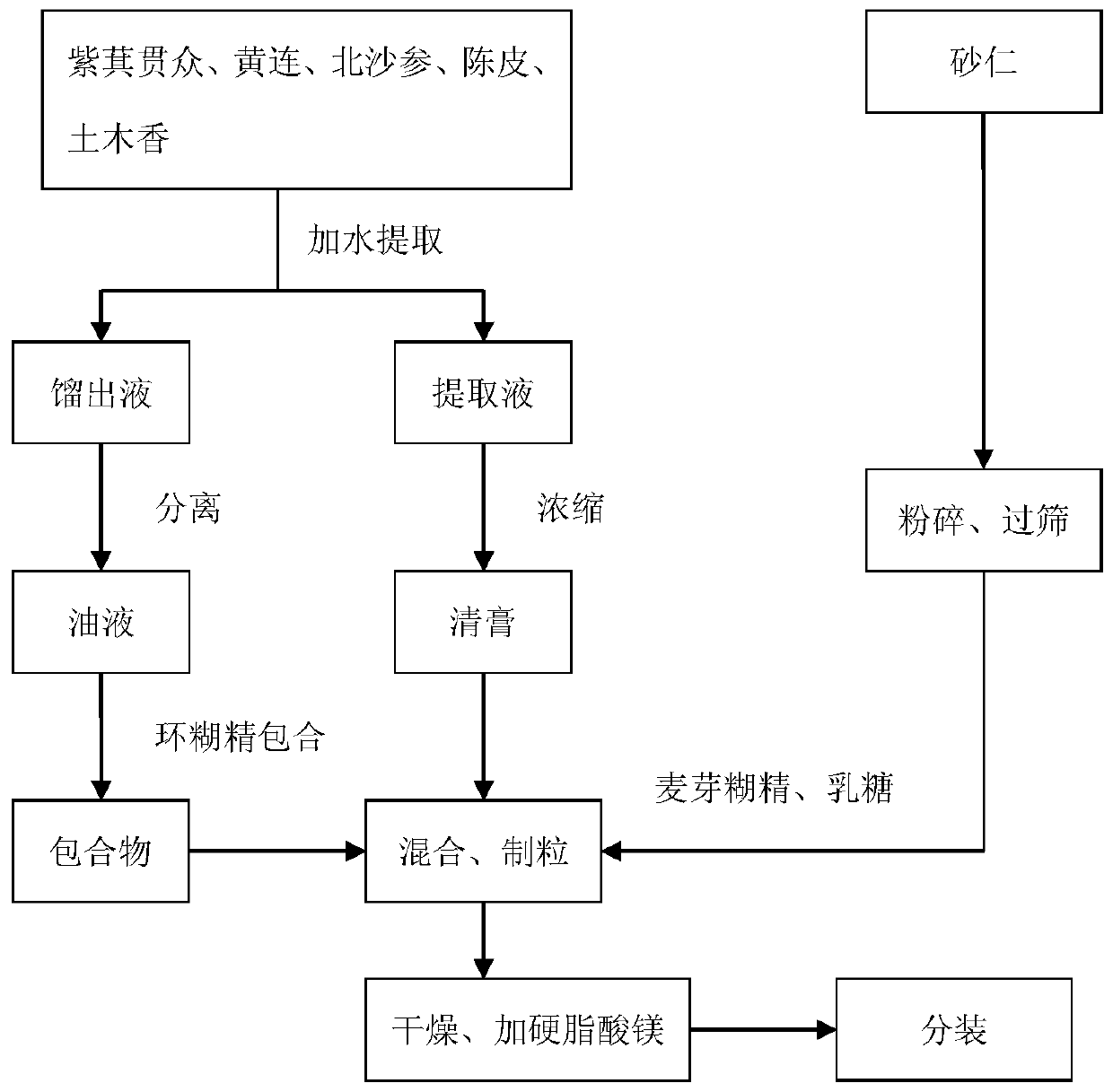
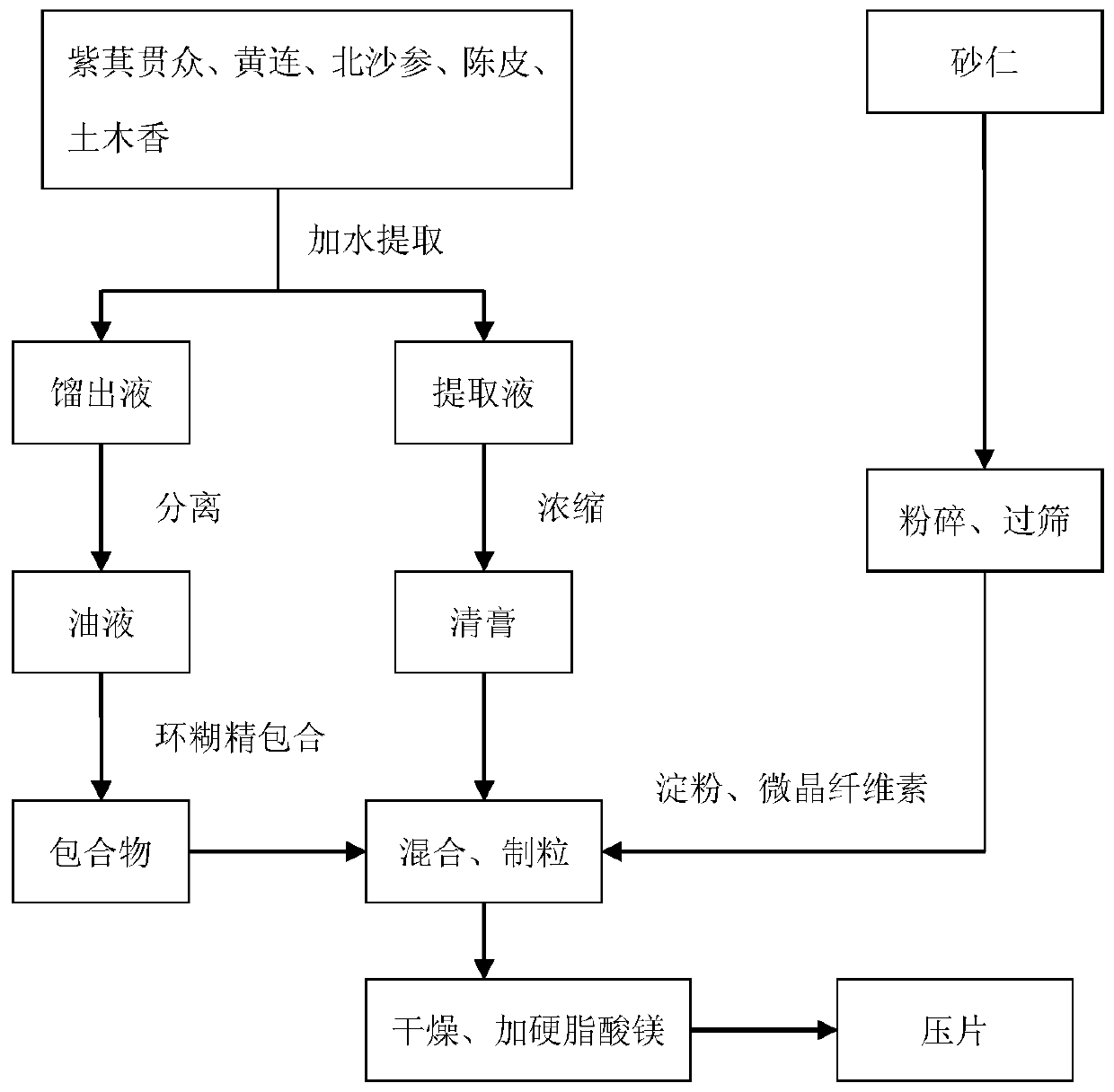
ActiveCN110269920AReduce medication burdenHigh active ingredientDigestive systemPteridophyta/filicophyta medical ingredientsAbdomen diseasesBULK ACTIVE INGREDIENT
The invention provides a traditional Chinese medicine mixture for relieving stomach diseases and a preparation method thereof. The traditional Chinese medicine mixture for relieving the stomach diseases is prepared from, by weight, 120 parts of Japanese flowering fern rhizome, 90 parts of coptis, 60 parts of fructus amomi, 60 parts of radix glehniae, 45 parts of pericarpium citri reticulatae and 45 parts of elecampane inula roots; the pharmaceutical form can be, but not limited to, capsules, granules and tablets; the preparation method mainly comprises the fives steps of pulverization, boiling, concentration, separation and preparation. The traditional Chinese medicine mixture in various pharmaceutical forms for relieving the stomach diseases is provided for a patient to choose and achieves the significant improvement of the active ingredients, and the dissolution rate of the active ingredients is greatly increased in an environment close to the stomach acid environment, which is beneficial to the absorption by the patient, the heavy metal content in Chinese patent medicine is obviously reduced, the stability is good, and the medication burden on the patient is reduced.
Owner:贵阳永乐药业有限公司
Ulva pertusa polysaccharide separation product and separation and purification method thereof
InactiveCN106749723AEfficient physiological activityEnhance blood lipid lowering effectPurification methodsIon exchange
The invention relates to the technical field of polysaccharide extraction and particularly relates to an ulva pertusa polysaccharide separation product which contains at least one of a separation product F1, a separation product F2 and a separation product F3. The separation product F1 is prepared from rhamnose, xylose and glucose according to a proportion of molar ratio of 1 : (0.29 to 0.35) : (0.13 to 0.15); the separation product F2 is prepared from rhamnose, xylose and glucose according to a proportion of molar ratio of 1 : (0.38 to 0.46) : (0.26 to 0.28); the separation product F3 is prepared from rhamnose and xylose according to a proportion of molar ratio of 1 : (0.36 to 0.44). The invention further relates to a separation and purification method of the separation product. The separation and purification method comprises the following steps of extracting ulva pertusa polysaccharide through a water extraction and alcohol precipitation method, performing ultrasonic dissolving treatment, performing centrifugal treatment, performing ion-exchange column chromatography treatment, performing dialysis treatment and freezing and drying to obtain the separation product F1, the separation product F2 and the separation product F3. The three separation products disclosed by the invention have high-efficiency antioxidant activity and establish base for researches on enhancing a blood fat reducing effect, reducing drug use burden of a patient and the like.
Owner:WEIFANG MEDICAL UNIV
Dexamethasone slow-release microsphere for injection in glass body
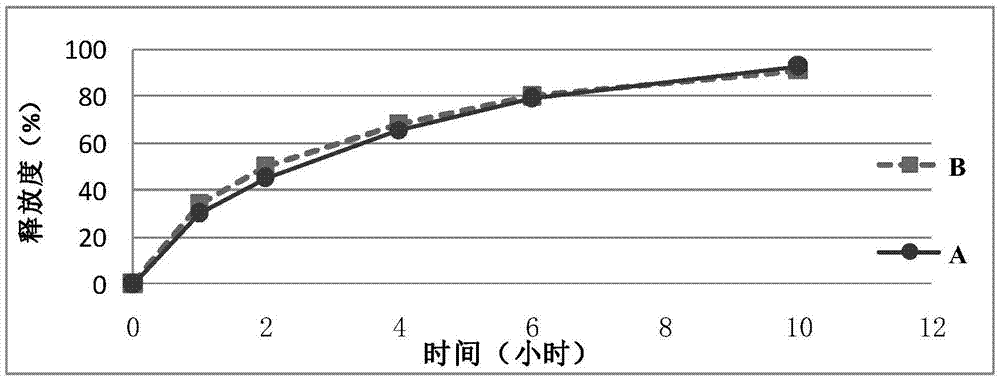
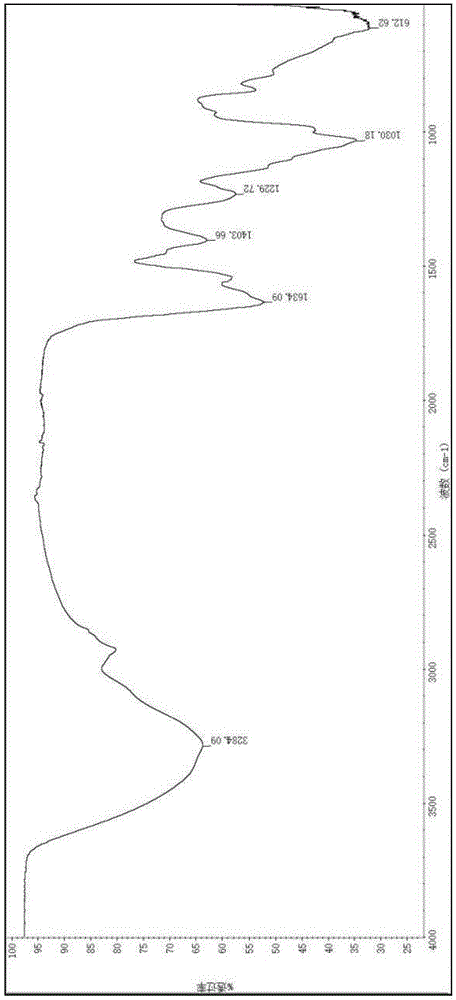
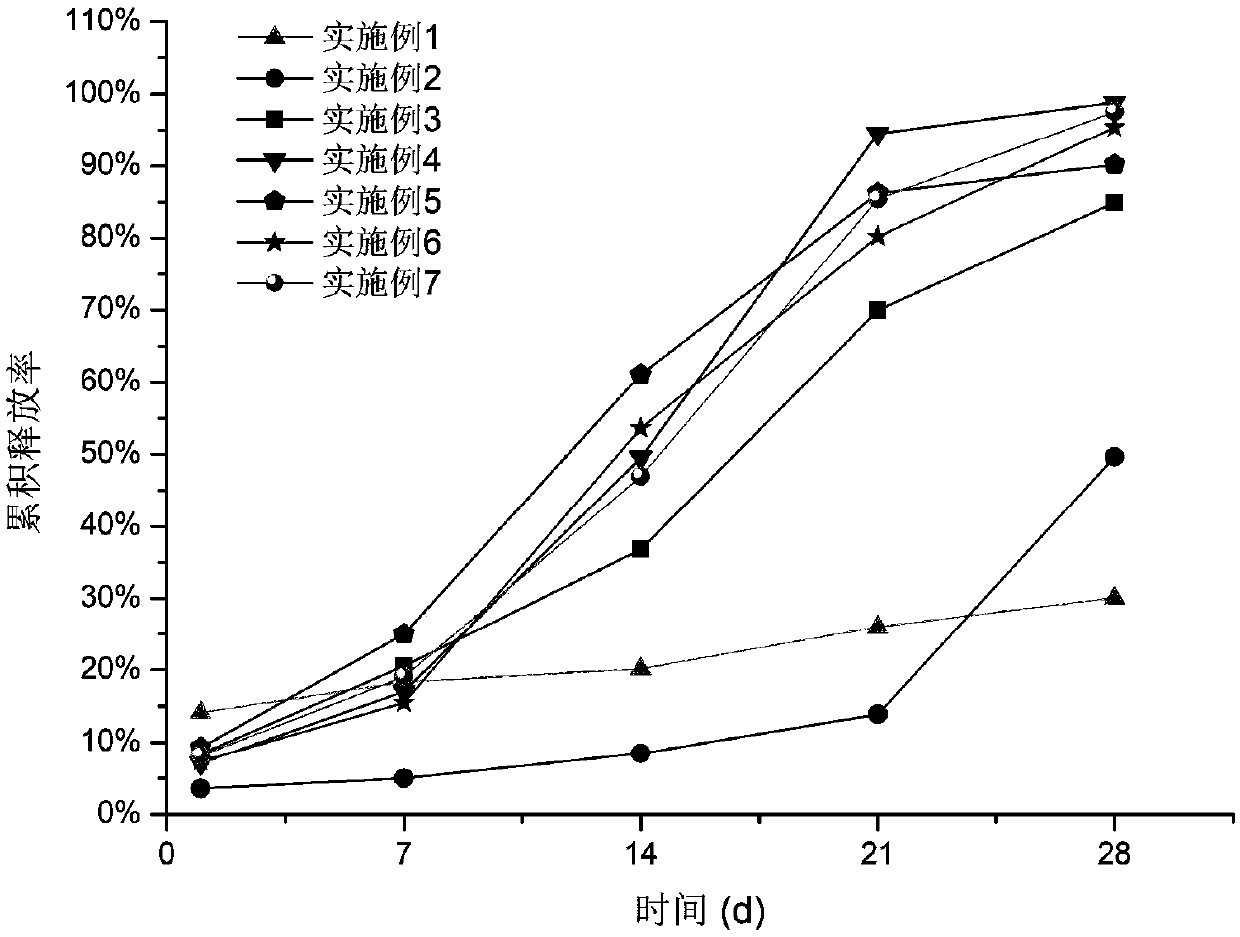
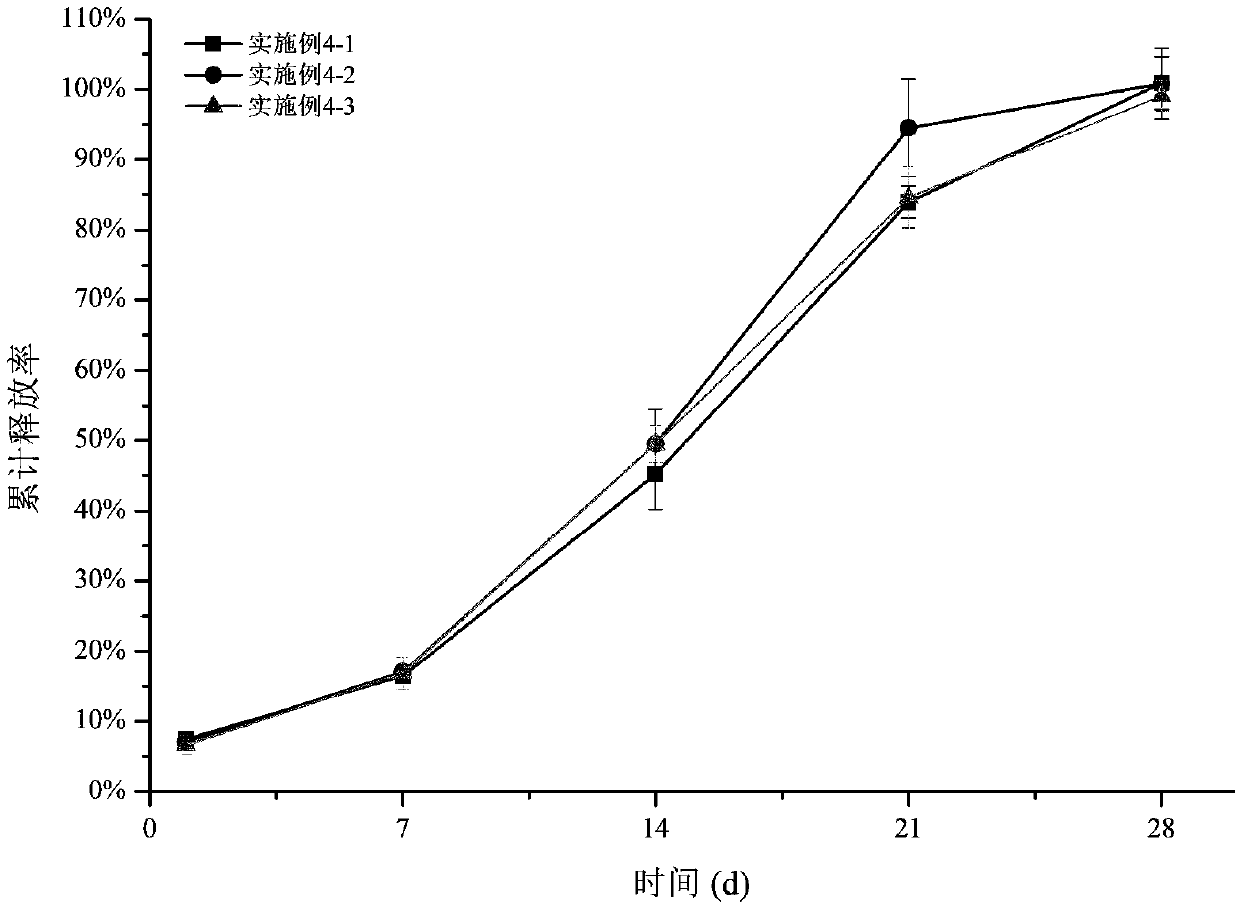
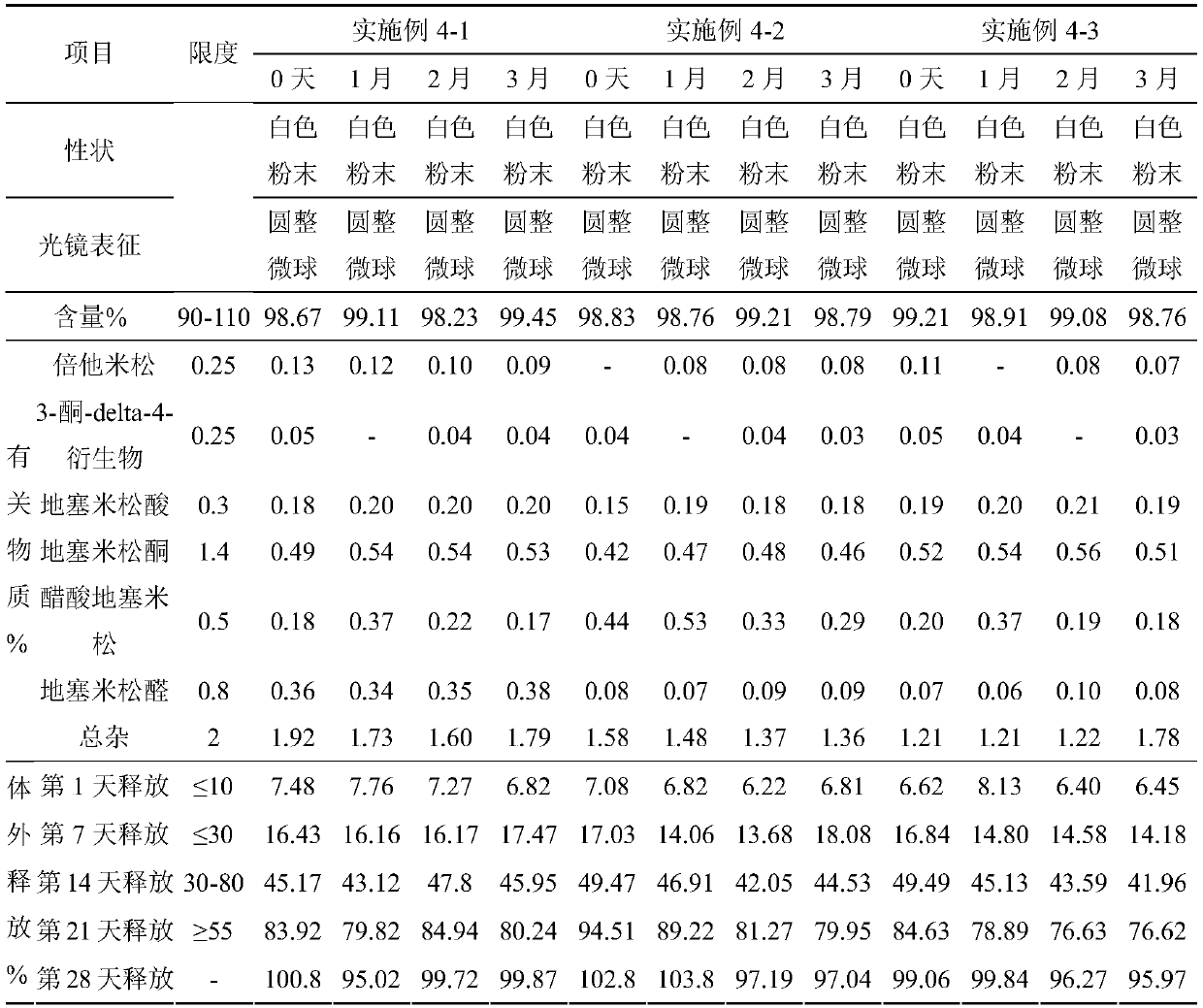
InactiveCN110384678AMaintenance drug concentrationReduce the frequency of medicationOrganic active ingredientsSenses disorderDexamethasoneMicrosphere
The invention relates to a dexamethasone slow-release microsphere for injection in a glass body. The microsphere contains dexamethasone and poly(lactide-co-glycolide). A solvent volatilizing method isadopted to prepare the microsphere, the preparation conditions are mild, the requirements for equipment are low, industrial production is easy, and a conventional injection needle can be used for completing administration in the glass body. The drug loading ratio of the prepared dexamethasone slow-release microsphere reaches 10-20%, the release behavior of the first-day release rate being less than or equal to 10%, the 7th day cumulative release rate being less than or equal to 30%, the 14th day cumulative release rate being 30% to 80% and the 21st day cumulative release rate being greater than or equal to 55% can be achieved, the effective treatment concentration is maintained for six months, through a stability test, it is shown that the quality characteristic is not changed significantly, the redissolving effect is good, and the microsphere is convenient for clinical application.
Owner:CHINA PHARM UNIV
Traditional Chinese medicine composition for treating allergic rhinitis
InactiveCN109718262ALittle side effectsReduce medication burdenRespiratory disorderImmunological disordersBudDrug
The invention relates to the technical field of traditional Chinese medicines, in particular to a traditional Chinese medicine composition for treating allergic rhinitis. The composition comprises siberian cocklebur herb, and also comprises optional one or more of winkled gianthyssop herb, dahurian angelica roots, biond magnolia flower-bud, forsythia fruits and baical skullcap roots. The traditional Chinese medicine composition for treating rhinitis is easy to absorb, has small side effect in use, can quickly and effectively treat nasal obstruction and rhinorrhea and keep smooth respiration, has quick effect and high cure rate for treating allergic rhinitis, greatly reduces the drug burden of patients, and is suitable for popularization and application.
Owner:王向军
Compound indigo pill
InactiveCN101040993AProlonged disintegration timeLong onset timePill deliveryDermatological disorderAlkanna tinctoriaSmoked Plum
The invention relates to a concentrated compound natural indigo pill which is prepared from the following raw materials: natural indigo 60g, purslane 200g, dahurian angelica root 100g, poria cocos 200g, alkanna tinctoria 80g, basket fern 60g, dandelion 80g, root of red rooted saliva 100g, rhizoma dioscoreae hypoglaueae 100g, Chinese dittany bark 100g, smoked plum 200g, schisandra fruit 100g, haw 60g and medicated leaven 60g.
Owner:焦林海
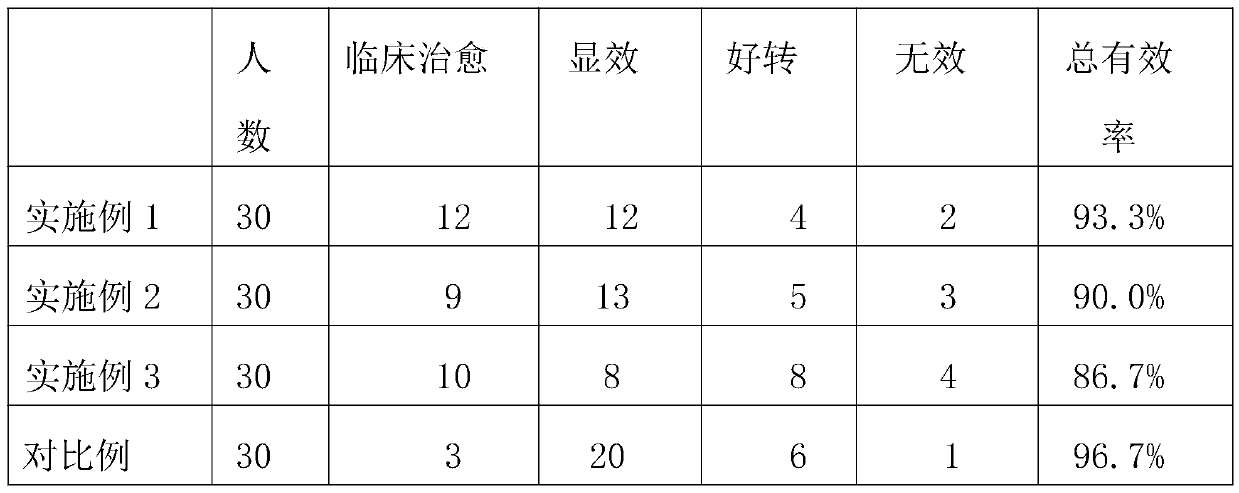
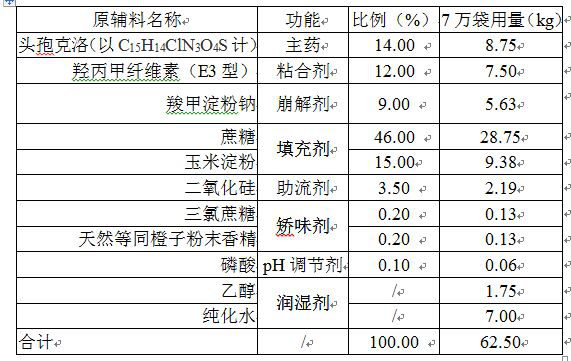
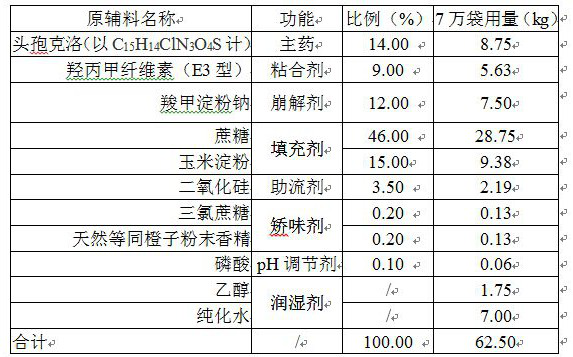
Cefaclor granule pharmaceutical composition
InactiveCN112137965ASolving the Bioequivalence DilemmaSolve the Equivalence DilemmaAntibacterial agentsOrganic active ingredientsSucrosePharmaceutical drug
The invention relates to a cefaclor granule pharmaceutical composition, and belongs to the technical field of pharmaceutical preparations. According to the technical scheme, the cefaclor granule composition contains cefaclor, corn starch, cane sugar, sodium carboxymethyl starch, silicon dioxide and hydroxypropyl methylcellulose, and the dosage of the hydroxypropyl methylcellulose and the dosage ofthe sodium carboxymethyl starch are not lower than 9% of the total weight of a unit dose product respectively. The mass ratio of the hydroxypropyl methylcellulose to the sodium carboxymethyl starch is 1: 1, the mass ratio of the sum of the dosages of the hydroxypropyl methylcellulose and the sodium carboxymethyl starch to the cefaclor (anhydrous) is (18-24): (12-16), and the viscosity of the hydroxypropyl methylcellulose is less than 100mPa.S (at 20 DEG C). The invention provides the cefaclor granule pharmaceutical composition which is stable in quality and biologically equivalent to originalresearch.
Owner:DISHA PHARMA GRP
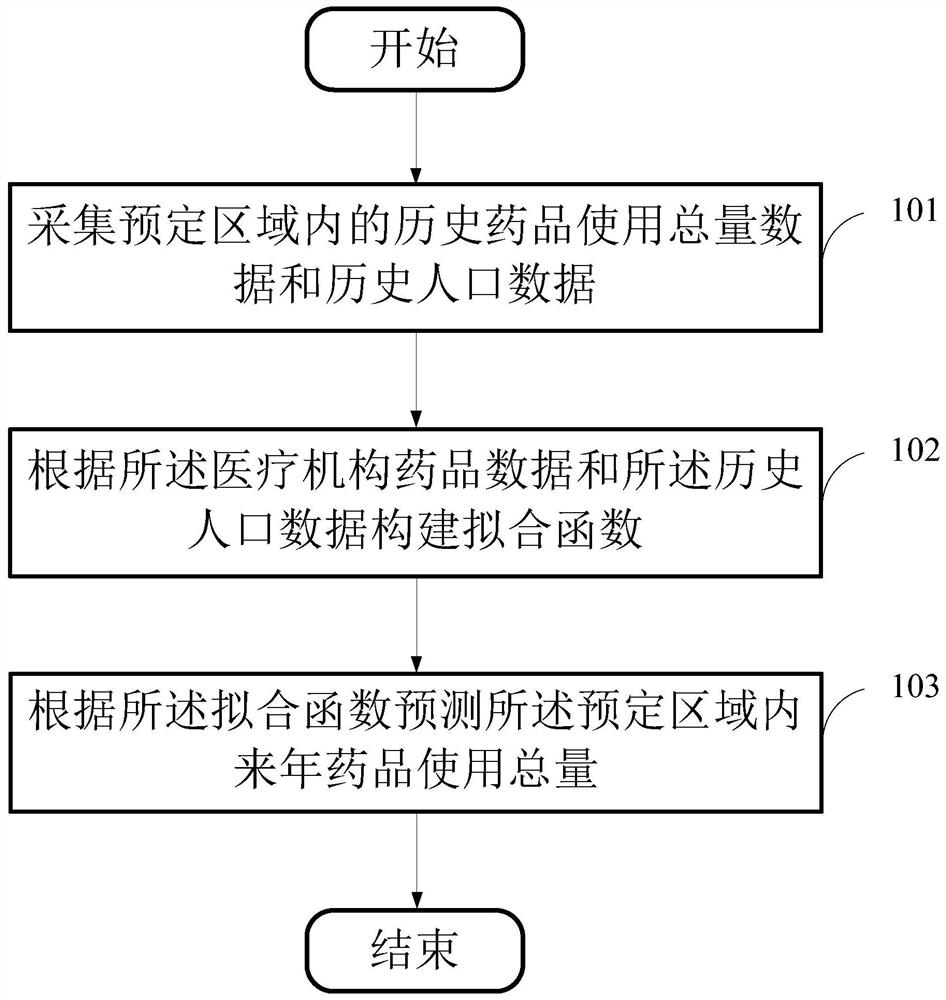
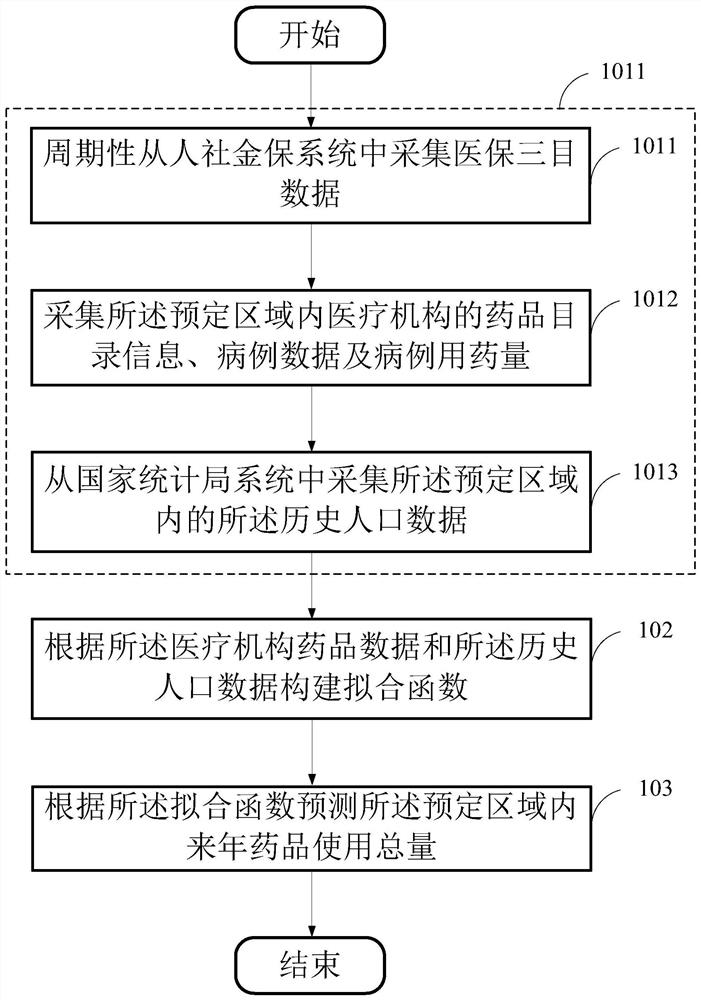
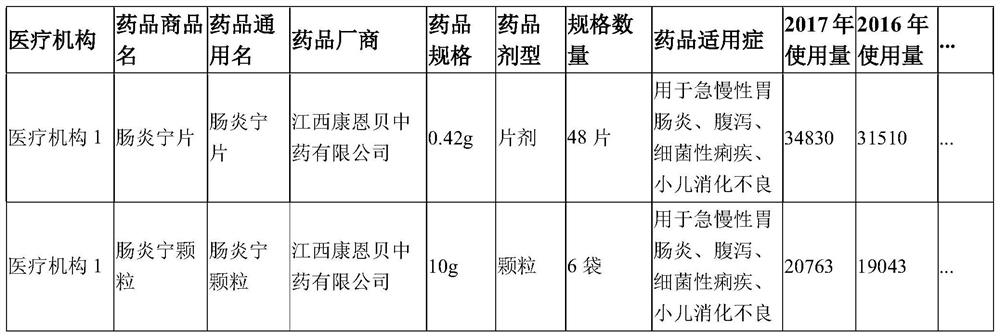
Drug inventory analysis method, storage medium and equipment based on medical big data
ActiveCN109524125BSolve procurement problemsSolve the problem of purchasing volumeDrug referencesEngineeringIntelligent management
The invention provides a medicine stock analysis method, a storage medium and device based on medical big data, wherein the method comprises the following steps of: acquiring medical institution medicine data and historical population data in a preset area; constructing a fitting function according to the medical institution medicine data and the historical population data; and predicting the total amount of the medicine used for the next year in the predetermined area according to the fitting function. The method provides data support for the medical institution management department and themedical institution, achieves accurate price negotiation between a medicine centralized purchasing platform and medicine enterprises, and also achieves intelligent management of medicine stock of themedical institution.
Owner:易保互联医疗信息科技(北京)有限公司
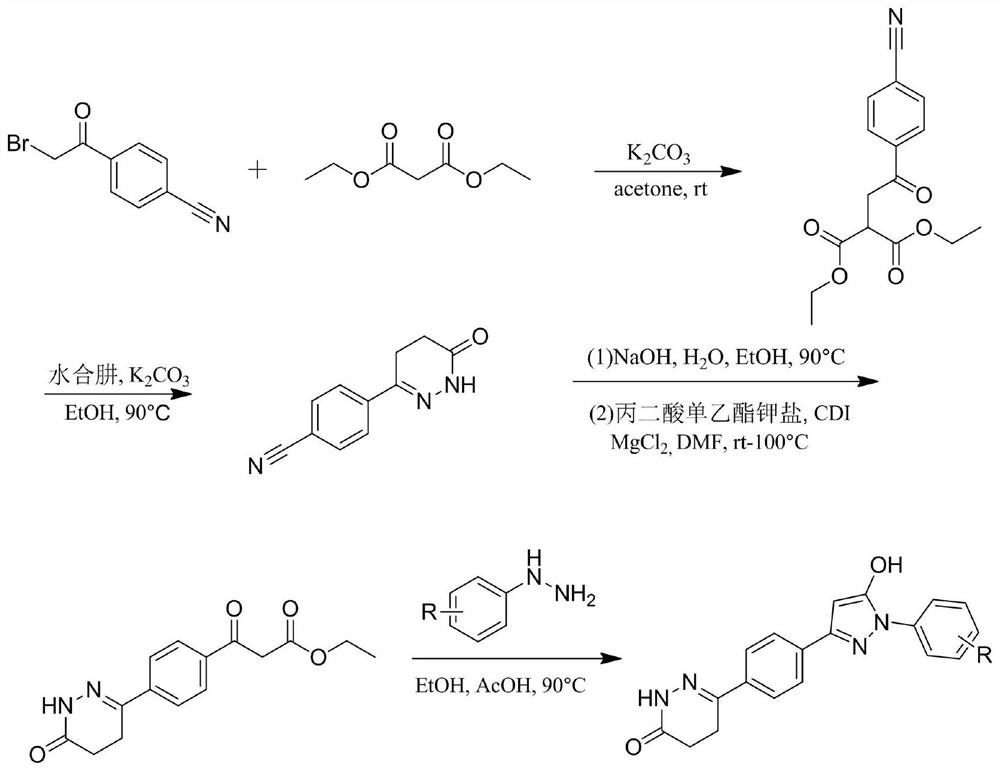
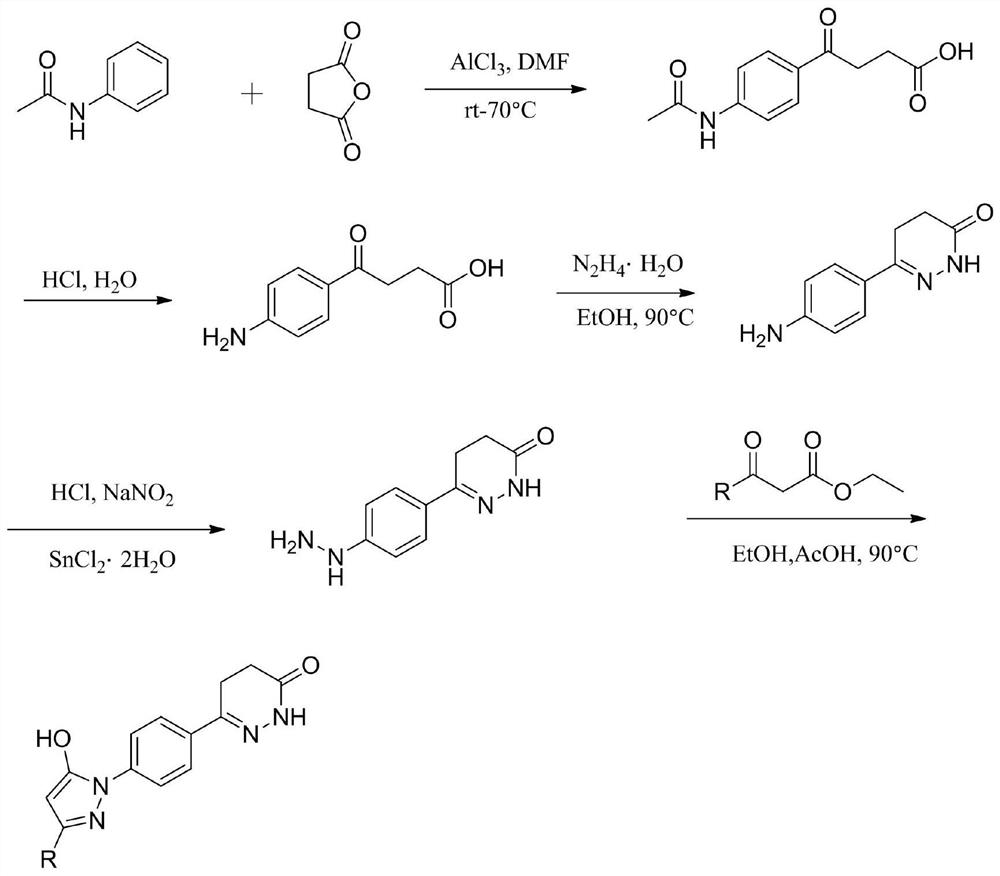
Pyrazol alcohol-pyridazinone coupling compound and pharmaceutical composition thereof and application of pyrazol pyridazinone-pyridazinone coupling compound in medicine
PendingCN113980001AExcellent anti-platelet aggregation effectImprove protectionOrganic active ingredientsNervous disorderDiseasePyridazine
The invention belongs to the technical field of pharmaceutical chemistry, and particularly relates to a pyrazole alcohol-pyridazinone coupling compound and a pharmaceutical composition thereof and application of the pyrazole alcohol-pyridazinone coupling compound in medicine. The pyrazole alcohol-pyridazinone coupled compound has dual action mechanisms of resisting platelet aggregation and protecting nerve cells, and is applied to preparation of medicines for preventing or treating cerebral apoplexy, cardiovascular and cerebrovascular diseases, senile dementia and complications thereof caused by thrombus and excessive free radicals.
Owner:GUIZHOU MEDICAL UNIV
A kind of traditional Chinese medicine composition for treating uremia and preparation method thereof
ActiveCN104740611BSignificant improvementNo toxicityPeptide/protein ingredientsUrinary disorderSide effectAlcohol
Owner:江西康宝医药生物科技有限公司
Traditional Chinese medicine used for treating uremia and preparation method thereof
InactiveCN103432467BNon-toxic and no side effectsEasy to manufactureUrinary disorderPlant ingredientsSide effectHouttuynia
The invention discloses a traditional Chinese medicine used for treating uremia and a preparation method thereof. The traditional Chinese medicine used for treating uremia comprises following raw materials, by weight:30 to 60 portions of fresh radix astragali, 10 to 30 portions of poria cocos peel, 10 to 30 portions of pericarpium arecae, 15 to 40 portions of exocarpium benincasae, 15 to 40 portions of semen plantaginis, 10 to 30 portions of fried fructus aurantii, 10 to 30 portions of roasted rhizoma atractylodis macrocephalae, 15 to 40 portions of rehmannia?root, 10 to 30 portions of cortex mori, 15 to 40 portions of cordate houttuynia, 15 to 40 portions of oldenlandia diffusa, 30 to 60 portions of serissa serissoides, 15 to 40 portions of lungwortlike lobaria herb, 30 to 60 portions of raw semen coicis, 10 to 30 portions of radix dipsaci, 10 to 30 portions of radix achyranthis bidentatae, 10 to 30 portions of yam rhizome, 10 to 30 portions of herba taxilli and 10 to 30 portions of rheum officinale. The results of more than 175 clinical experiments show that: effective rate of the traditional Chinese medicine in treatment of uremia reaches to 70%, and 30% of the patients treated by using the traditional Chinese medicine are on the mend obviously. No toxic or side effect is caused by the traditional Chinese medicine, and the traditional Chinese medicine is capable of improving uremia significantly. In addition, the traditional Chinese medicine is low in cost, is easy to prepare, can be popularized in the folk, and is capable of reducing medicine burden of patients.
Owner:张启锐 +2
Bone-recovering tablets
ActiveCN1742898AGood slow release effectReduce dosageSkeletal disorderPill deliveryMedicineActive ingredient
The present invention discloses a Gukang tablet and its preparation method. It is made up by using Chinese medicinal materials of psoralea seed, banana stump, dipsacus root, notoginseng and creeping wood sorrel as raw material through a certain preparation process.
Owner:贵州维康子帆药业股份有限公司
Bone-recovering tablets
ActiveCN1298357CGood slow release effectReduce dosageSkeletal disorderPill deliveryMedicineActive ingredient
The present invention discloses a Gukang tablet and its preparation method. It is made up by using Chinese medicinal materials of psoralea seed, banana stump, dipsacus root, notoginseng and creeping wood sorrel as raw material through a certain preparation process.
Owner:贵州维康子帆药业股份有限公司
Compound pesticide composition containing spirodiclofen and tolfenpyrad and preparation thereof
InactiveCN103518732AReduce the dosageReduce medication burdenBiocideAnimal repellantsIndoxacarbCOMPONENT II
The invention discloses a compound pesticide composition containing spirodiclofen. The effective components comprise a component I and a component II, wherein the component I is spirodiclofen; the component II is one of indoxacarb, chlorantraniliprole, tolfenpyrad, chromafenozide, pyridalyl, flubendiamide and chlorfluazuron; and the mass ratio of the component I to the component II is 40:1-1:40. The invention also discloses an agriculturally applicable preparation formulation prepared from the composition and application of the composition in preventing and treating crop pests. The compound pesticide containing spirodiclofen has an obvious synergistic action, is used for preventing and treating pests on fruit trees, cotton, wheat, vegetables, flowers, rice and other crops, and especially has an obvious effect on preventing and treating Lepidoptera pests and acarid pests.
Owner:YONGNONG BIOSCI
A kind of ganglioside sustained-release tablet and preparation method thereof
ActiveCN108815131BReasonable prescriptionImprove stabilityOrganic active ingredientsNervous disorderProlonged-release tabletPharmaceutical drug
The invention discloses a ganglioside sustained release tablet and a preparation method thereof. The ganglioside sustained release tablet is prepared from monosialoganglioside and specific pharmaceutical auxiliary materials through a specific method. The process is simple, the cost is low, the medicine price can be effectively reduced, and the burden of a patient is relieved. Meanwhile, the prepared sustained release tablet is good in sustained-release effect, the patient can carry medicine at any time for medicine supplementing, and the sustained-release tablet is convenient to use.
Owner:四川奇格曼药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com