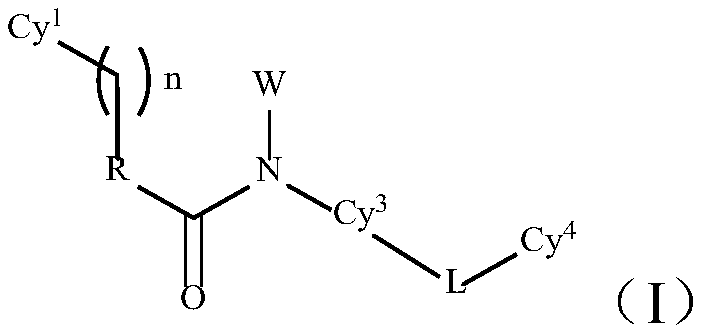
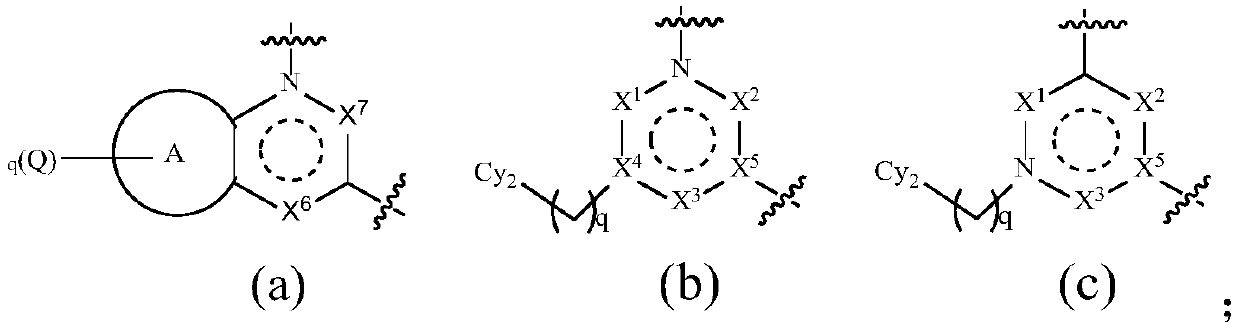
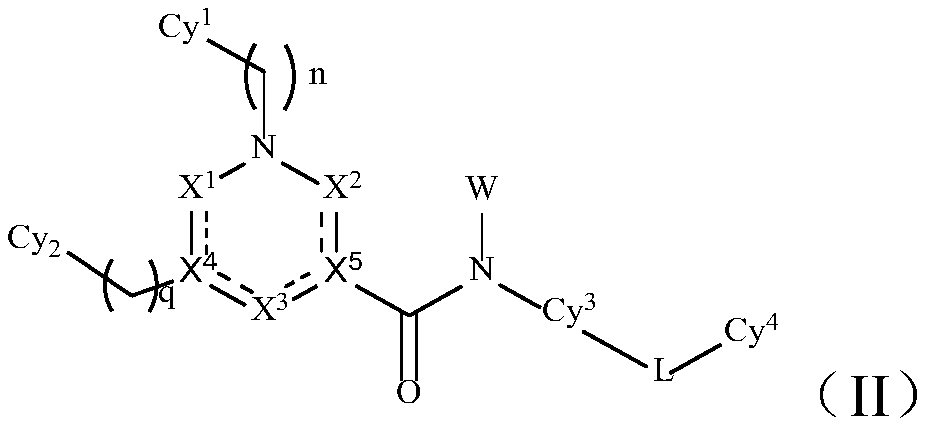
TAM family kinase and/or CSF1R kinase inhibitor and application thereof
A substituent and selected technology, applied in the field of medicine, can solve the problems of inhibitors on the market and achieve the effect of inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0373] Example 1: Synthesis of 4-oxo-1-((tetrahydro-2H-pyran-4-yl)methyl)-5-(p-tolyl)-1,4-dihydropyridine-3-carboxylic acid
[0374]
[0375] step
[0376]
[0377] Step 1: Synthesis of 2-(p-tolyl)acetyl chloride
[0378]
[0379]Under nitrogen protection, 2-(p-tolyl)acetic acid (25g, 0.17mol, 1.0eq) was added to dichloromethane (250mL), and oxalyl chloride (42.25g, 0.34mol, 2.0eq) was added dropwise at room temperature, and then Add DMF (0.25mL), and reflux reaction for 1h after dropping. Concentrate to remove the solvent, add dichloromethane (100 mL) to redissolve, and concentrate again (repeated twice). The obtained crude product is directly used in the next reaction without purification.
[0380] Step 2: Synthesis of 2,2-dimethyl-5-(2-(p-tolyl)acetyl)-1,3-dioxane-4,6-dione
[0381]
[0382] Add 2,2-dimethyl-1,3-dioxane-4,6-dione (36g, 0.25mol, 1.05eq), pyridine (31g, 0.39mol, 2.3eq) into dichloromethane (350mL ), add the acid chloride prepared in the step d...
Embodiment 2
[0397] Example 2 N-(5-((6,7-dimethoxyquinolin-4-yl)oxy)pyridin-2-yl)-4-oxo-1-((tetrahydro-2H-pyridine Synthesis of pyran-4-yl)methyl)-5-(p-tolyl)-1,4-dihydropyridine-3-carboxamide (compound 1)
[0398]
[0399] step:
[0400]
[0401] Step 1: Synthesis of 4-((6-bromopyridin-3-yl)oxy)-6,7-dimethoxyquinoline
[0402]
[0403] 6-bromopyridin-3-ol (1.74g, 10.0mmol, 1.0eq), 4-chloro-6,7-dimethoxybenzopyridine (2.24g, 10.0mmol, 1.0eq) and 4-dimethyl Aminopyridine (3.67g, 30.0mmol, 3.0eq) was dissolved in toluene (50mL), heated to 100°C for 16 hours. After the reaction was detected by LC-MS, the reaction solution was directly concentrated, and the crude product was purified by silica gel column chromatography (DCM:MeOH=50:1~20:1) to obtain a white solid 4-((6-bromopyridin-3-yl)oxy base)-6,7-dimethoxyquinoline (1.70g, yield: 47%)
[0404] Step 2: Synthesis of 5-((6,7-dimethoxyquinolin-4-yl)oxy)pyridin-2-amine
[0405]
[0406] 4-((6-bromopyridin-3-yl)oxy)-6,7-dimethox...
Embodiment 3
[0412] Example 3: Synthesis of intermediate 4-oxo-5-(p-tolyl)-1,4-dihydropyridazine-3-carboxylic acid ethyl ester hydrochloride
[0413]
[0414] Step 1: Synthesis of 2-(p-tolyl)acetyl chloride
[0415]
[0416]Dissolve 2-(p-tolyl)acetic acid (9.0g, 60mmol, 1.0eq) in DCM (90mL), add oxalyl chloride (15.2g, 120mmol, 2.0eq) dropwise at room temperature, after the addition is complete, add DMF dropwise (0.1 mL), heated to reflux for 2 hours. After cooling to room temperature, the reaction solution was concentrated to obtain the product (10 g crude product), which was directly used in the next reaction.
[0417] Step 2: Synthesis of ethyl 2-diazo-3-oxo-4-(p-tolyl)butyrate
[0418]
[0419] The intermediate 2-(p-tolyl)acetyl chloride (10g crude product, 60mmol, 1.0eq) was added into a three-necked flask, cooled to 0°C, and ethyl diazoacetate (13.7g, 120mmol, 2.0eq) was slowly added dropwise. After the dropwise addition was completed, return to room temperature and stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com